Ziconotide injection hypodermic implant and preparation method thereof
A ziconotide, injection-type technology, applied in anti-inflammatory agents, pharmaceutical formulations, peptide/protein components, etc., can solve the problems of unstable product emulsification effect, affecting the sustained release effect of preparations, and sudden drug release. The effect of easy control of drug dose, complete encapsulation, and small loss of activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
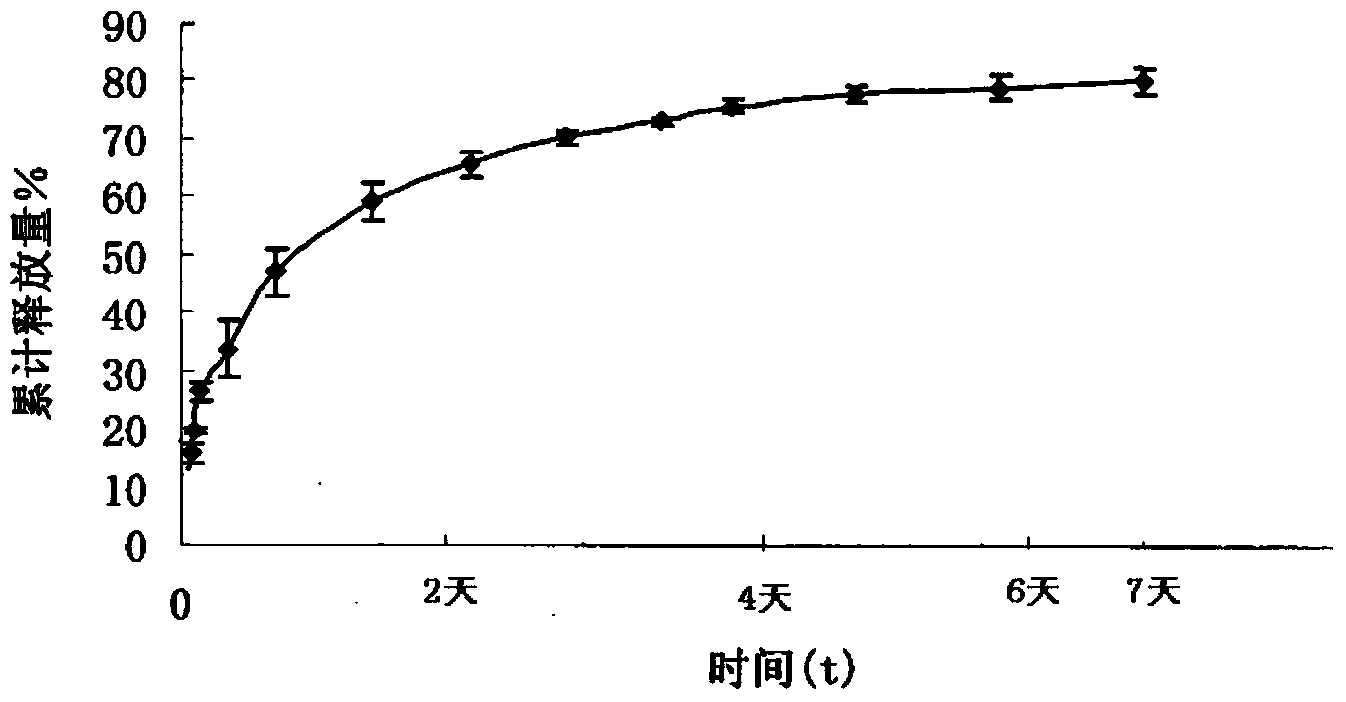
[0035] Example 1: Preparation of Ziconotide Injectable Subcutaneous Implant
[0036] 1. Prescription (1000 sticks)
[0037]
[0038] 2. Preparation of Ziconotide Microcapsules with PLGA as Skeleton
[0039] Dissolve PLGA (Mn=20000, LA:GA=50:50) completely in dichloromethane to prepare 2% PLGA dichloromethane solution, and disperse ziconotide in 2% PLGA dichloromethane solution , formulated into a solution containing ziconotide, and spray-dried at 25°C under a pressure of 0.2 MPa to obtain microcapsules.
[0040] 3. Preparation of temperature-sensitive hydrogel
[0041] Mix Poloxamer 407 with 1ml of water for injection at a low temperature of 20°C, let it stand still to fully swell to a solution state, and make a hydrogel solution.
[0042] 4. Preparation of Ziconotide Injectable Subcutaneous Implant
[0043] Add the ziconotide microcapsule powder into the hydrogel solution, mix evenly, add mannitol and freeze-dry to prepare 1000 ziconotide injection-type subcutaneous im...
Embodiment 2
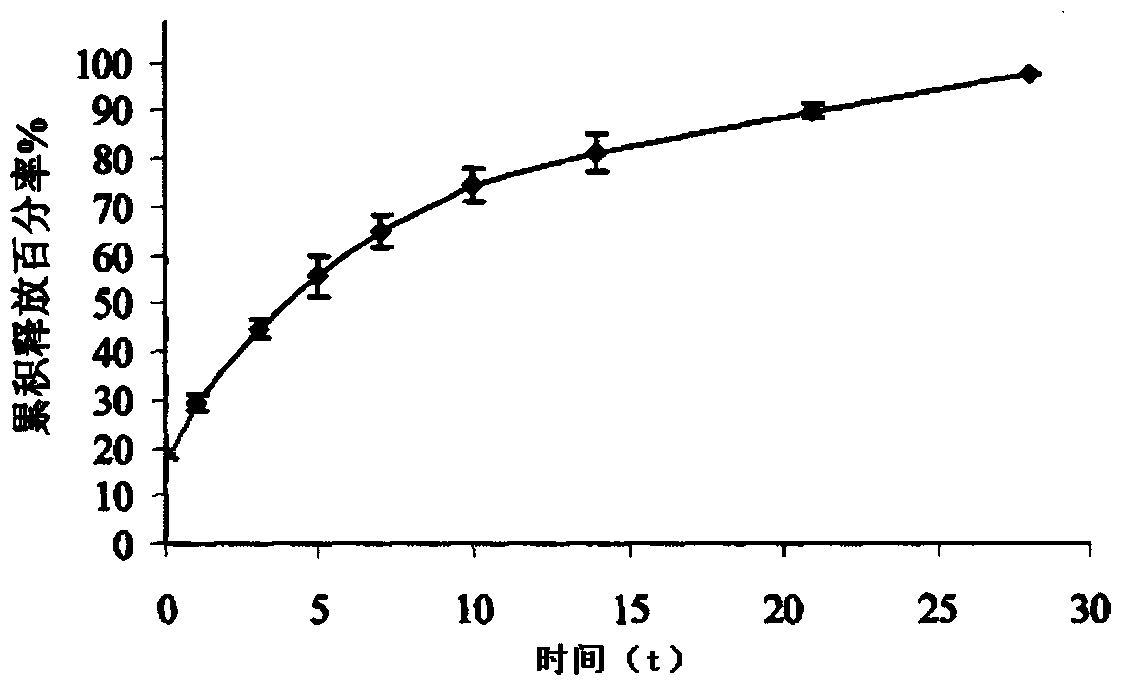
[0045] Example 2: Preparation of Ziconotide Injectable Subcutaneous Implant
[0046] 1. Prescription (1000 sticks)
[0047]
[0048] 2. Preparation of Ziconotide Microcapsules with PLGA as Skeleton
[0049] Completely dissolve PLGA (Mn=20000, LA:GA=50:50) in chloroform to prepare a 10% PLGA chloroform solution, disperse ziconotide in 10% PLGA chloroform solution, and make a preparation containing ziconotide Notide solution, freeze-dried to obtain microcapsules.
[0050] 3. Preparation of temperature-sensitive hydrogel
[0051] Mix PLGA-PEG-PLGA (molecular weight: 5000) with 1ml of water for injection at a low temperature of 15°C, let it stand still to fully swell to a solution state, and make a hydrogel solution.
[0052] 4. Preparation of Ziconotide Injectable Subcutaneous Implant
[0053] Add the ziconotide microcapsule powder into the hydrogel solution, mix evenly, add mannitol and freeze-dry to prepare 1000 ziconotide injection-type subcutaneous implants.
[0054] Be...
Embodiment 3
[0055] Example 3: Preparation of Ziconotide Injectable Subcutaneous Implant
[0056] 1. Prescription (1000 sticks)
[0057]
[0058] 2. Preparation of Ziconotide Microcapsules with PLGA as Skeleton
[0059] Dissolve PLGA (Mn=10000, LA:GA=75:25) completely in acetone to prepare a 20% PLGA acetone solution, disperse Ziconotide in 20% PLGA acetone solution, and prepare Ziconotide Notide solution was spray-dried at 25°C and 3MPa pressure to prepare microcapsules.
[0060] 3. Preparation of temperature-sensitive hydrogel
[0061] Mix PLGA-PEG-PLGA (molecular weight: 4000) with 1ml of water for injection at a low temperature of 15°C, let it stand still to fully swell to a solution state, and make a hydrogel solution.
[0062] 4. Preparation of Ziconotide Injectable Subcutaneous Implant
[0063] Add the ziconotide microcapsule powder into the hydrogel solution, mix evenly, add mannitol and freeze-dry to prepare 1000 ziconotide injection-type subcutaneous implants.
[0064] Be...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com