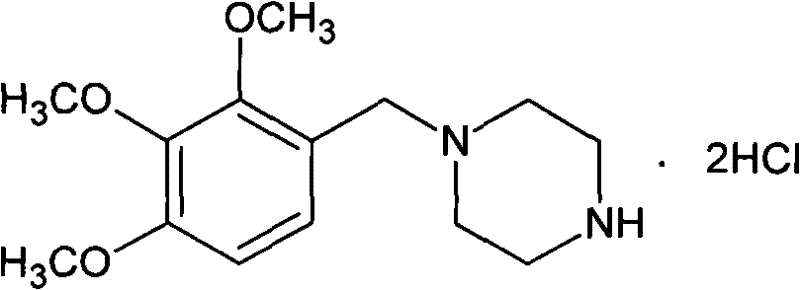
Method for preparing trimetazidine hydrochloride
A technology of trimetazidine hydrochloride and piperazine, applied in the field of medicinal chemistry, to achieve high yield, overcome low product purity and good quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
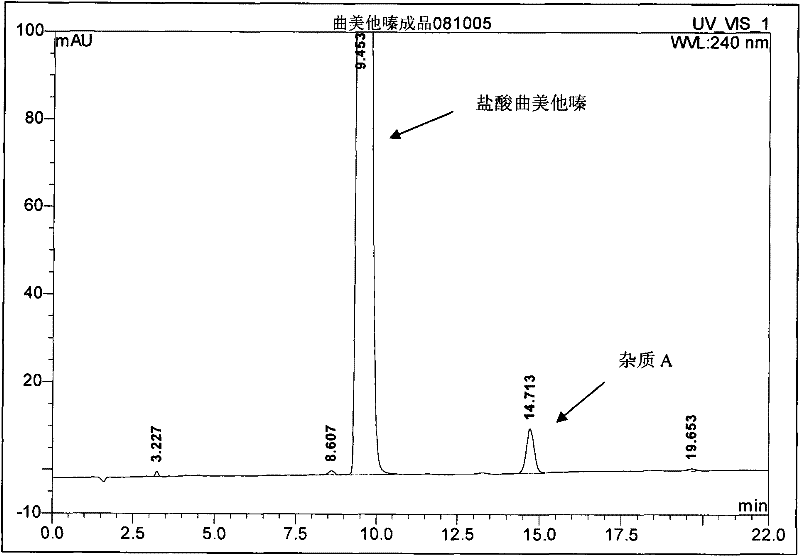
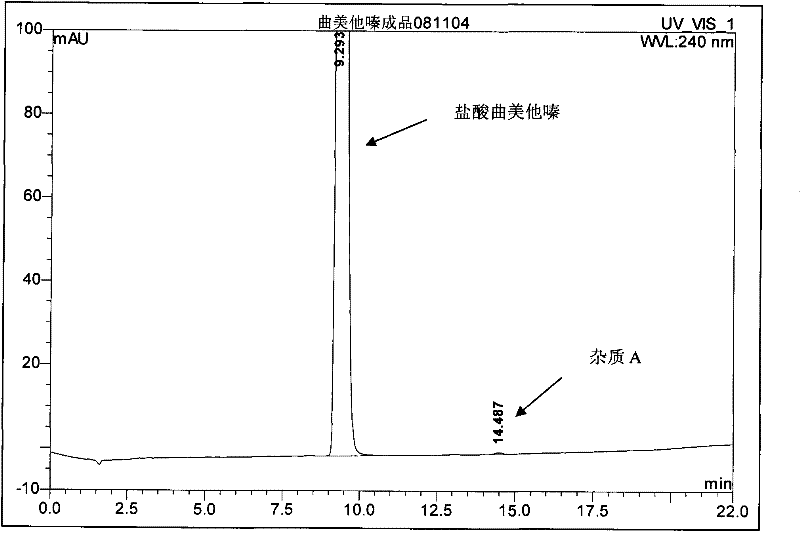
[0042] 196 grams (1.0 moles) of 2,3,4-trimethoxybenzaldehyde, 344 grams (4.0 moles) of anhydrous piperazine, 10 grams of 10% palladium carbon catalyst, 2700 milliliters of water and 300 milliliters of ethanol are dropped into hydrogenation kettle, Airtight, after purging the system with nitrogen, keep the hydrogen pressure at 0.8~1.2MPa, stir the reaction at 70-75°C, after absorbing hydrogen for 1.5 hours, the reactant no longer absorbs hydrogen, cool down, release the pressure, filter to remove palladium carbon, and the filtrate After concentration, add 500 ml of dichloromethane, filter to remove piperazine, and concentrate the filtrate under reduced pressure to obtain a light yellow oil, then add 2000 ml of ethanol, hydrochloric acid to adjust the pH to 1-2, and filter to obtain the crude product of trimetazidine hydrochloride, a mixed solvent of ethanol and water After recrystallization, 275.2 g (0.81 mol) of trimetazidine hydrochloride was obtained, with a yield of 81% and ...
Embodiment 2
[0044] 196 grams (1.0 moles) of 2,3,4-trimethoxybenzaldehyde, 172 grams (2.0 moles) of anhydrous piperazine, 10 grams of 10% palladium carbon catalyst, 2040 milliliters of water and 360 milliliters of ethanol are dropped into the hydrogenation kettle, The hydrogenation and post-treatment methods were the same as in Example 1 to obtain 247.3 g (0.73 moles) of the product, with a yield of 73% and a purity of 99.59% (HPLC).
Embodiment 3
[0046] 196 grams (1.0 moles) of 2,3,4-trimethoxybenzaldehyde, 258 grams (3.0 moles) of anhydrous piperazine, 10 grams of 10% palladium carbon catalyst, 2560 milliliters of water and 240 milliliters of ethanol are dropped into the hydrogenation kettle, The hydrogenation and post-treatment methods were the same as in Example 1 to obtain 264.9 g (0.78 moles) of the product, with a yield of 78% and a purity of 99.63% (HPLC).
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com