Trimetazidine hydrochloride tablet and preparation method thereof
A technology of trimetazidine hydrochloride and trimetazidine hydrochloride, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of unknown absorption, distribution and excretion, and the inability to predict the clinical therapeutic effect and other issues, to achieve the effects of reducing production costs, being suitable for large-scale production, and being easy to prepare
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
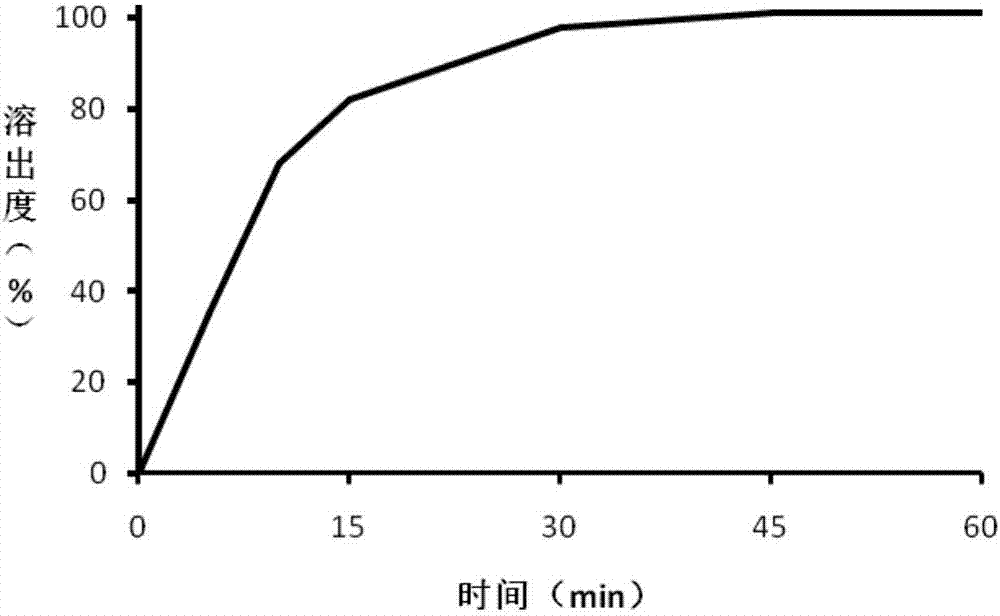
Embodiment 1
[0037] The trimetazidine hydrochloride coated tablet of the present embodiment, prescription is:
[0038]
[0039] Preparation Process:
[0040]Trimetazidine hydrochloride is passed through a 60 mesh sieve, and mannitol is passed through a 50 mesh sieve to prepare trimetazidine hydrochloride after sieving and mannitol after sieving, and take trimetazidine hydrochloride 20Kg after sieving, Cornstarch 28kg, 4.5Kg povidone K120 and 35.15kg mannitol after sieving to obtain mixed material A, mixed material A is added in the hopper of the wet mixing granulator, the pot cover is opened, the stirring I speed button is opened, and mixed for 180s Finally, turn on the II speed button, mix for 180s, and mix evenly to obtain the mixed material B, then turn on the I speed of stirring, chop the II speed, open the feeding slurry valve on the wet mixing granulator, and wet mixing at the speed of the peristaltic pump at 60rpm Under the condition that the atomization pressure of the granulat...
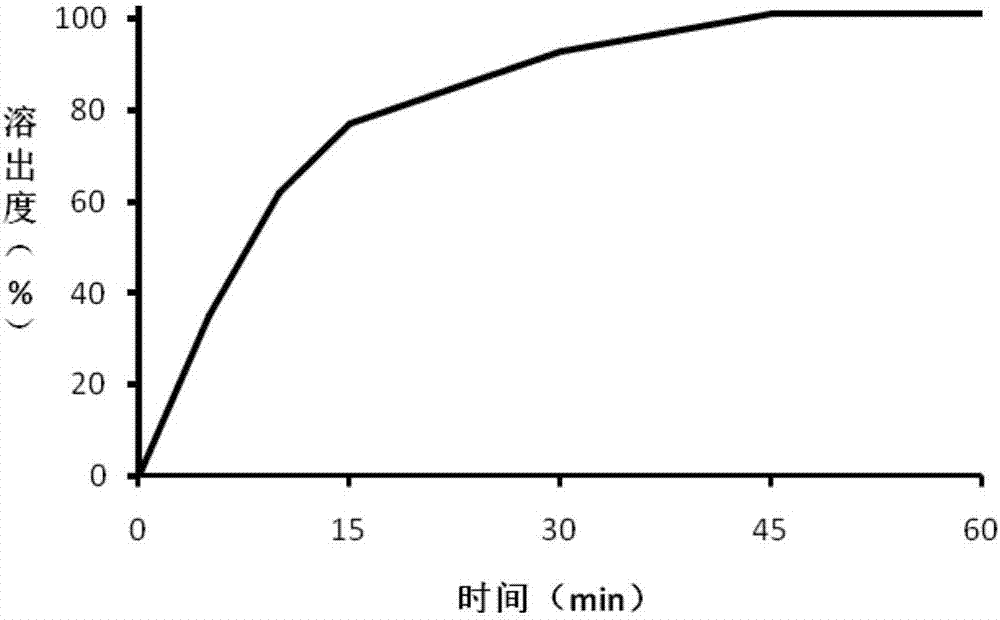
Embodiment 2
[0044] The trimetazidine hydrochloride coated tablet of the present embodiment, prescription is:
[0045]
[0046] The preparation process is:
[0047] Trimetazidine hydrochloride is passed through a 60-mesh sieve, and mannitol is passed through a 50-mesh sieve to prepare trimetazidine hydrochloride after sieving and mannitol after sieving, and take trimetazidine hydrochloride 18Kg after sieving, Cornstarch 25kg, 5Kg povidone K120 and 37kg of mannitol after sieving obtain mixed material A, mixed material A is added in the hopper of wet mixing granulator, open pot cover, open stirring I speed button, after mixing 180s, Turn on the speed II button, mix for 180s, and mix evenly to obtain the mixed material B, then turn on the stirring speed I, chop the speed II, open the feeding slurry valve on the wet mixing granulator, the speed of the peristaltic pump is 60rpm, and the atomization pressure is 0.3 Under the condition of Mpa, 8Kg of purified water is added, and the control t...
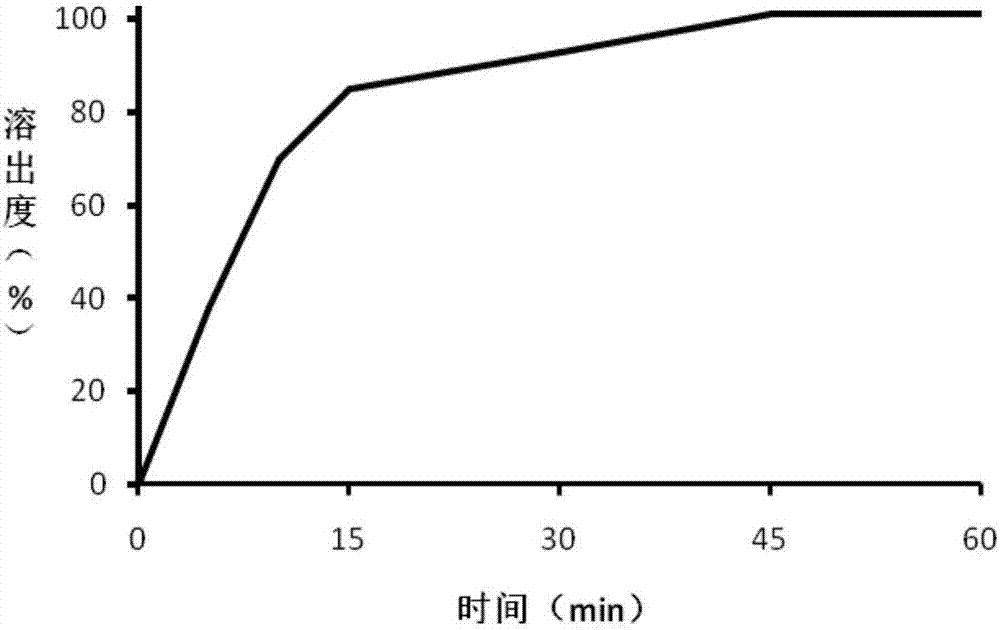
Embodiment 3
[0051] The trimetazidine hydrochloride coated tablet of the present embodiment, prescription is:
[0052]
[0053]
[0054] Preparation Process:
[0055] Trimetazidine hydrochloride is passed through a 60 mesh sieve, and mannitol is passed through a 50 mesh sieve to prepare trimetazidine hydrochloride after sieving and mannitol after sieving, and take trimetazidine hydrochloride 22Kg after sieving, Cornstarch 30kg, 4.0Kg povidone K120 and 33kg of mannitol after sieving to obtain mixed material A, mixed material A is added in the hopper of the wet mixing granulator, the pot cover is opened, the stirring I speed button is turned on, and after mixing for 180s , turn on the button of speed II, mix for 180s, mix evenly to obtain the mixed material B, then turn on the speed of stirring I, chop the speed II, open the feeding slurry valve on the wet mixing granulator, and the speed of the peristaltic pump is 60rpm, wet mixing granules Under the condition of machine atomization ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com