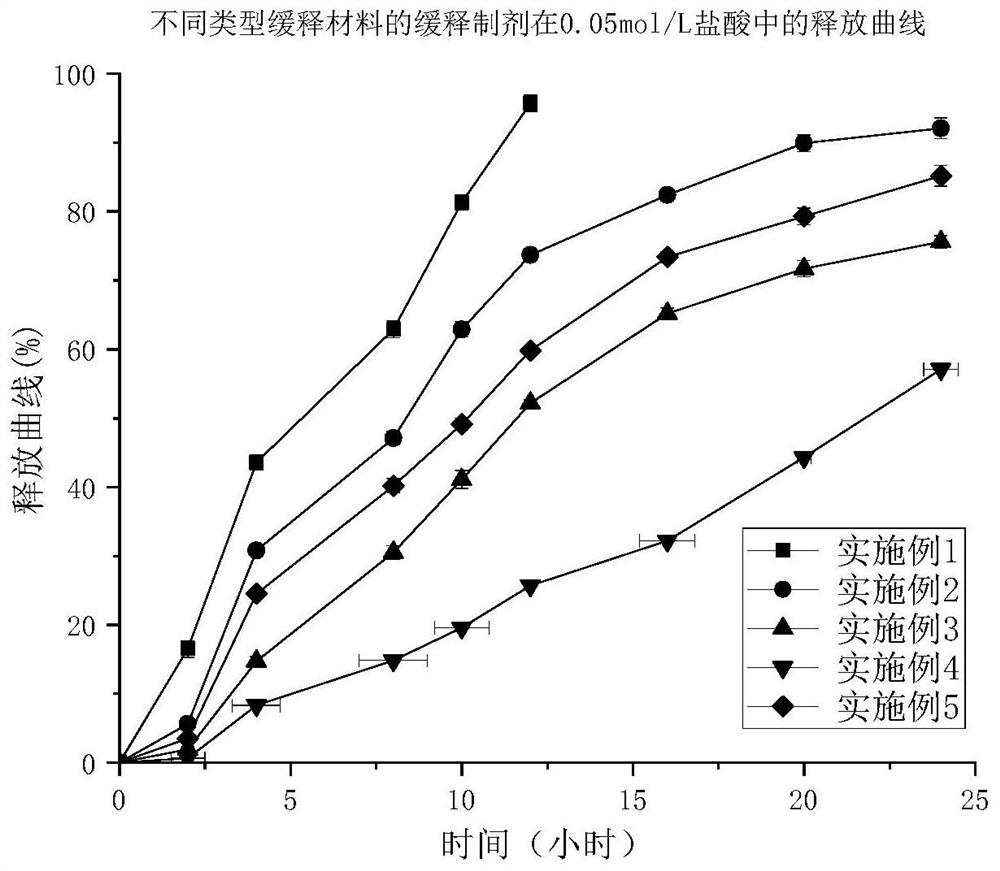
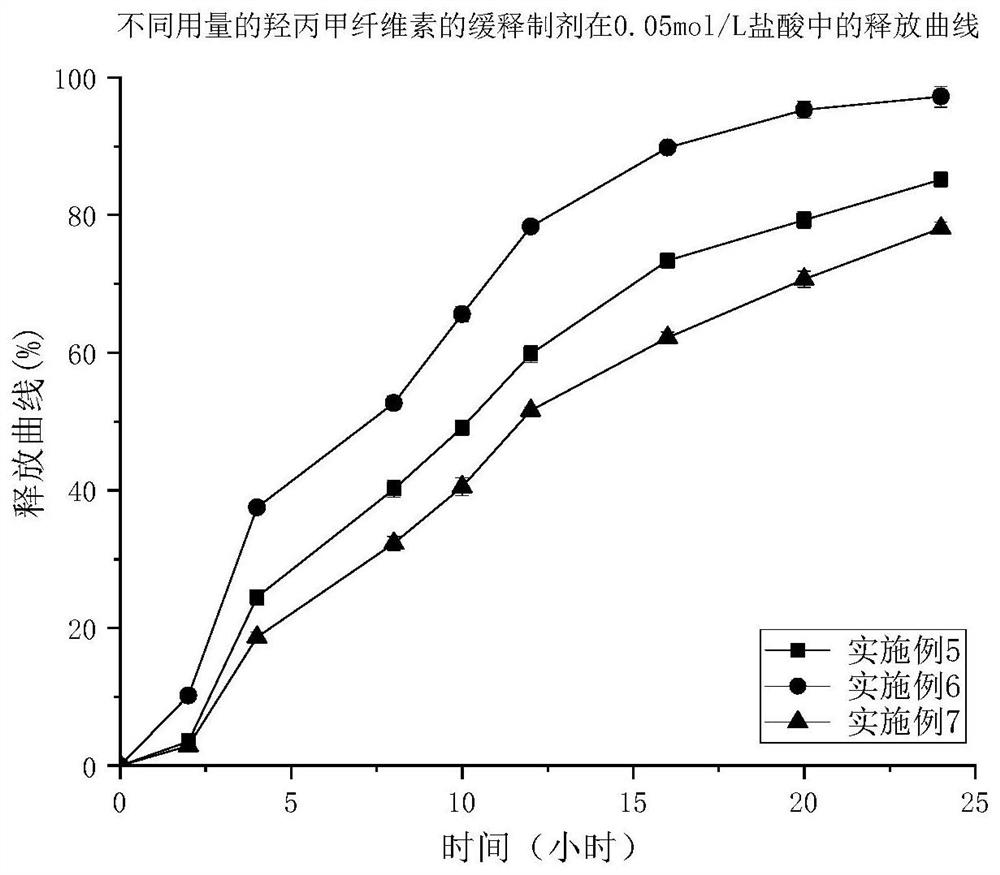
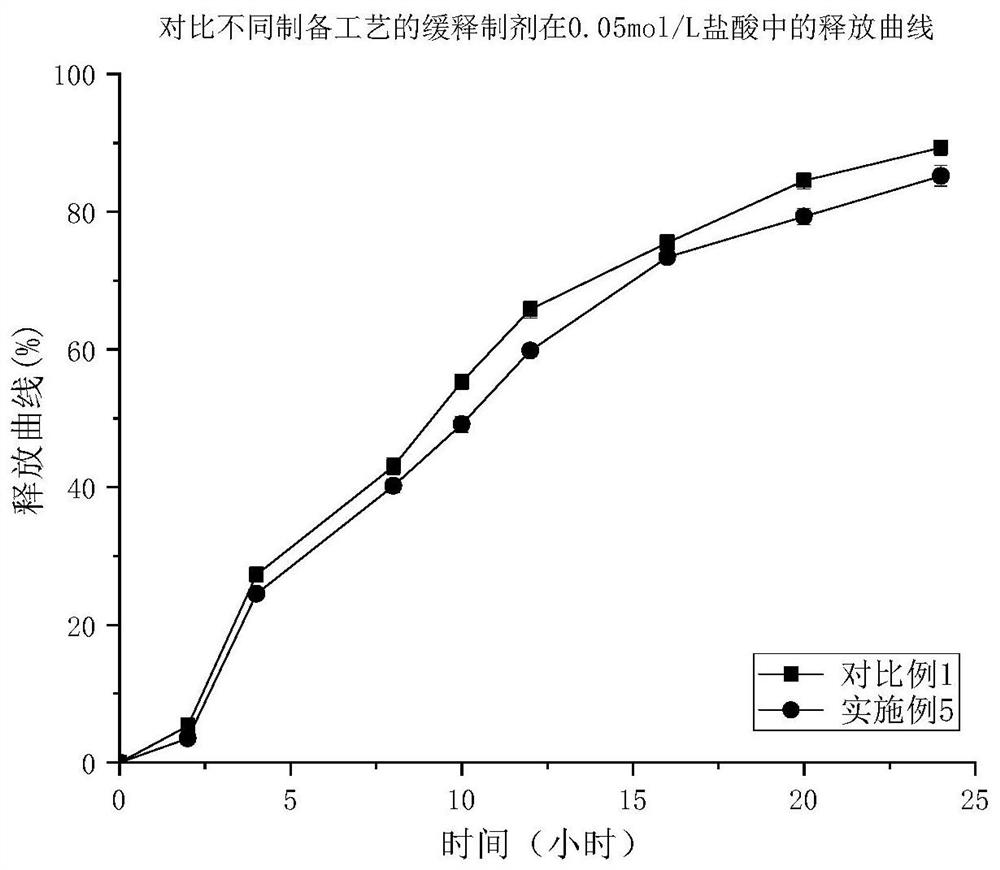
A kind of trimetazidine hydrochloride sustained-release preparation and preparation method thereof
A technology of trimetazidine hydrochloride and sustained-release preparations, which is applied in the fields of anti-toxic agents, pill delivery, and pharmaceutical formulations, and can solve problems such as cumbersome operations, long time-consuming, and complicated processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Prescription composition Prescription volume 1000 tablets / g Viblitizine hydrochloride 80 corn starch 20 Juweitone K30 5 Hydroxyl cellulose 39 Magnesium stearate 1
[0017] Preparation process: Add hydrochloride, polyhone K30, and corn starch to the wet -made particle cooker body, turn on the mixing knife and cut cutter for 10min pre -mixing. Wet wet -fired granules; after the purification water is sprayed into the pellets for 3 minutes; the obtained wet particles are wet, and then put in the fluidized bed for drying. The amount of mixed -release materials is mixed for 20 minutes, and then the prescribed amount of magnesium magnesium is added evenly for 5 minutes. Finally, the mixed material is transferred to the high -speed rotary compressor for pressure, which is the finished tablet.
Embodiment 2
[0019] Prescription composition Prescription volume 1000 tablets / g Viblitizine hydrochloride 80 corn starch 20 Juweitone K30 5 Polyette oxygenne 303 39 Magnesium stearate 1
[0020] Slow -release materials use polyethylene oxylene 303, and other prescription composition and preparation processes 1.
Embodiment 3
[0022] Prescription composition Prescription volume 1000 tablets / g Viblitizine hydrochloride 80 corn starch 20 Juweitone K30 5 Sodium alginate 39 Magnesium stearate 1
[0023] The mitigation material uses sodium algina, and other prescription composition and preparation process are the same as the embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com