Trimetazidine hydrochloride sustained-release preparation and preparation method thereof
A technology of trimetazidine hydrochloride and sustained-release preparations, which is applied in the fields of anti-toxic agents, pill delivery, and pharmaceutical formulations, and can solve problems such as low yield, uneasy control, and increased failure rate of pellets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
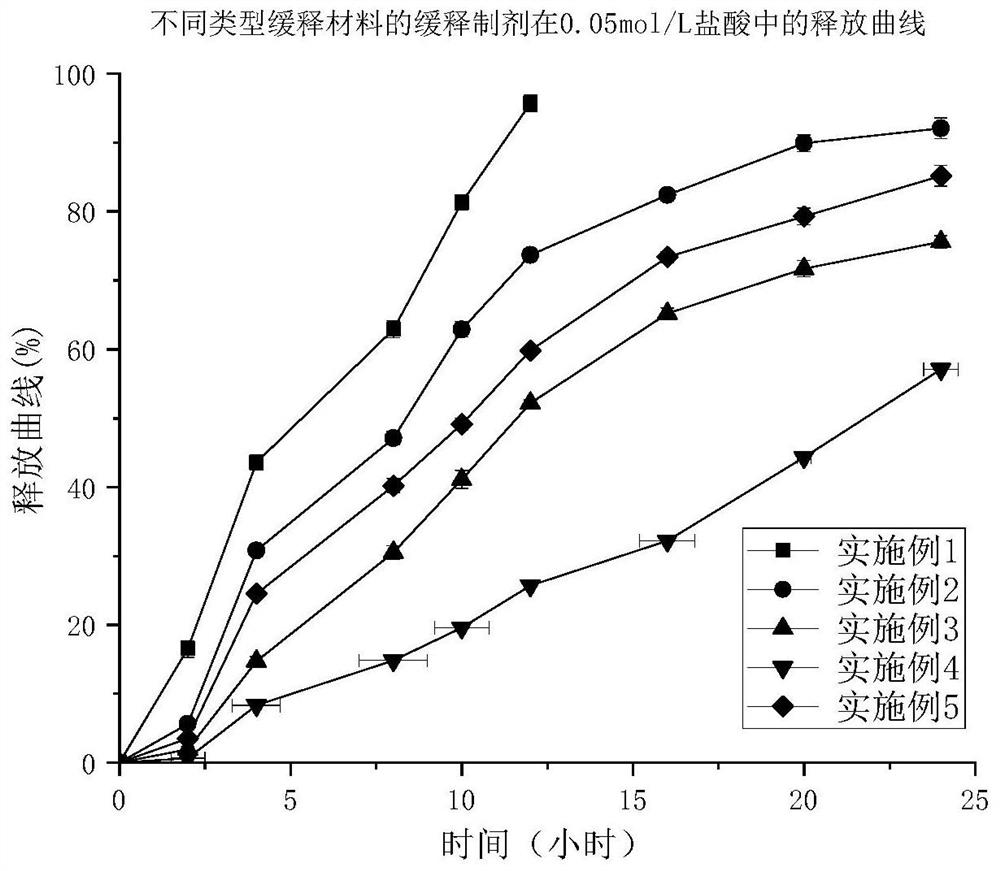
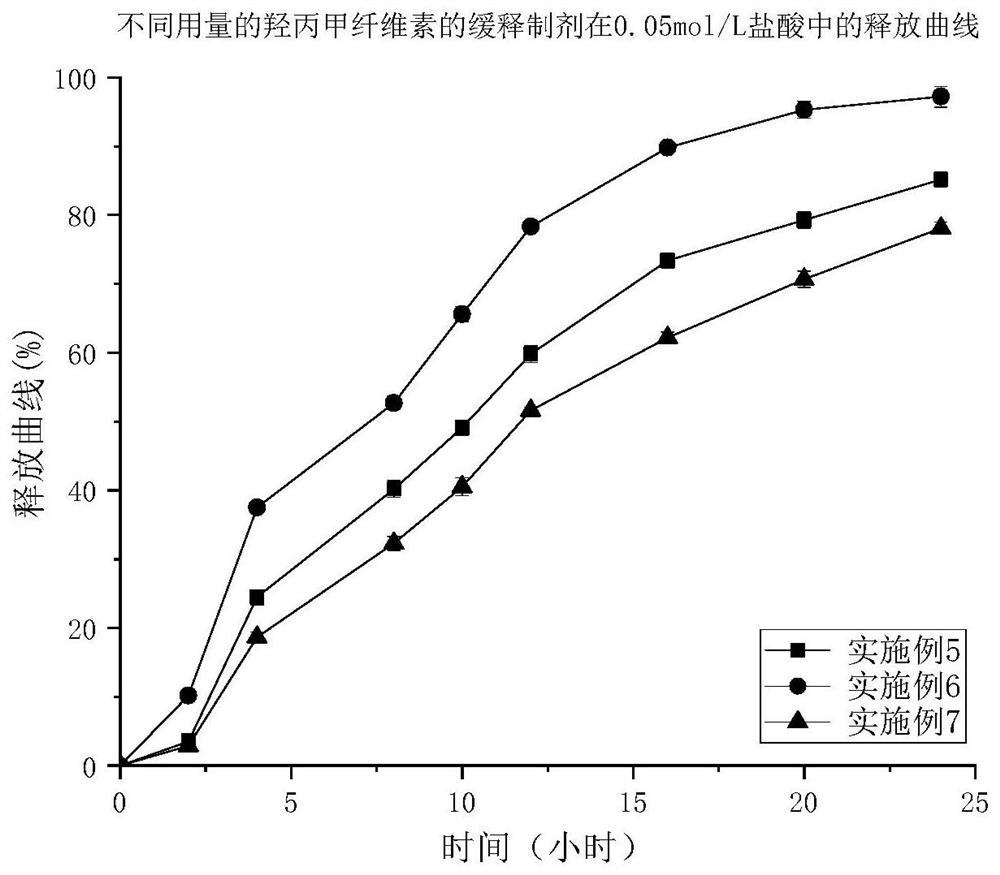
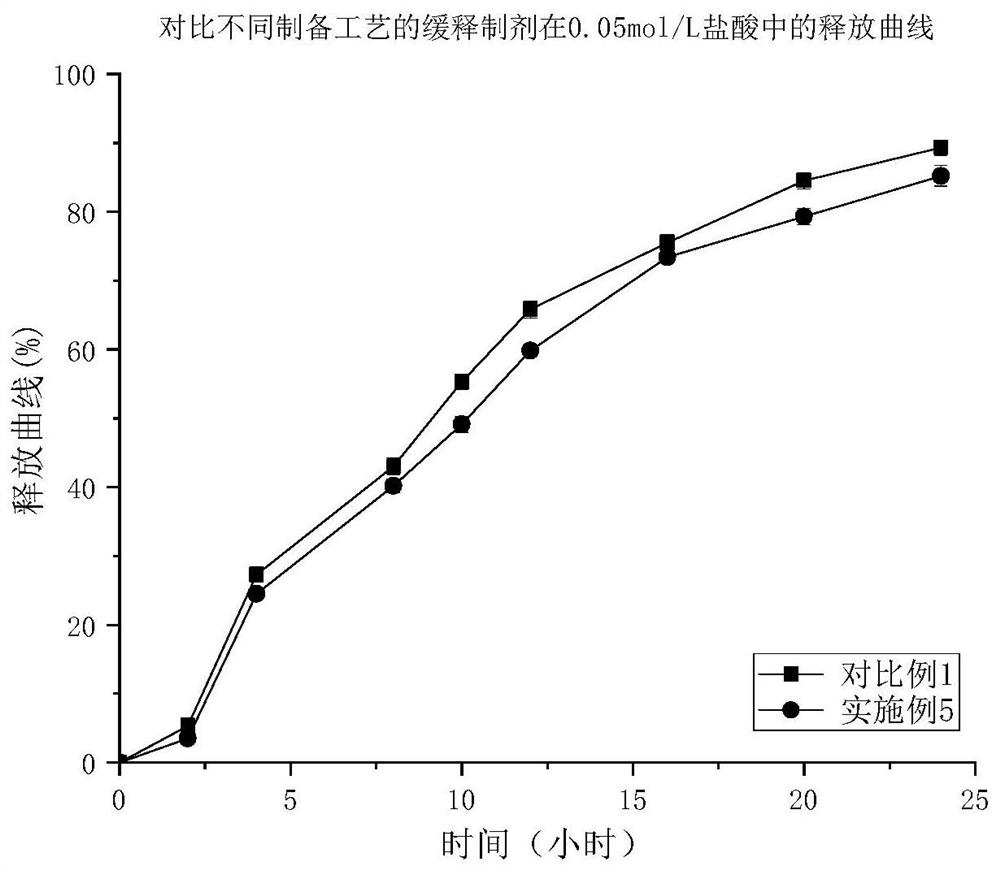
Image
Examples
Embodiment 1
[0016] prescription composition Prescription quantity 1000 tablets / g trimetazidine hydrochloride 80 corn starch 20 Povidone K30 5 Hydroxypropyl Cellulose 39 Magnesium stearate 1
[0017] Preparation process: Add trimetazidine hydrochloride, povidone K30, and corn starch into the pot of the wet granulator, turn on the stirring knife and cutting knife for 10 minutes of premixing, after the mixing is completed, turn on the stirring knife and cutting knife and spray into purified water Carry out wet granulation; continue to granulate for 3 minutes after the purified water is sprayed; wet granules are wet granulated, and then put into a fluidized bed for drying, and the dried granules are dry granulated; the dry granules are mixed with the prescription A certain amount of sustained-release material was mixed for 20 minutes, then the prescribed amount of magnesium stearate was added evenly for 5 minutes, and finally the mixed material was...
Embodiment 2
[0019] prescription composition Prescription quantity 1000 tablets / g trimetazidine hydrochloride 80 corn starch 20 Povidone K30 5 Polyethylene oxide 303 39 Magnesium stearate 1
[0020] The sustained-release material is polyethylene oxide 303, and the composition and preparation process of other prescriptions are the same as in Example 1.
Embodiment 3
[0022] prescription composition Prescription quantity 1000 tablets / g trimetazidine hydrochloride 80 corn starch 20 Povidone K30 5 sodium alginate 39 Magnesium stearate 1
[0023] Sodium alginate is used as the slow-release material, and the composition and preparation process of other prescriptions are the same as those in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com