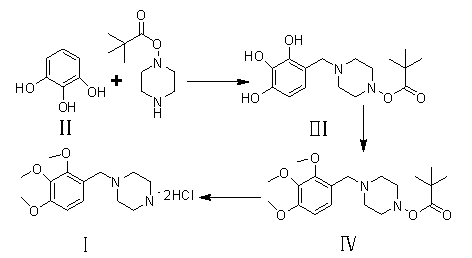
Novel synthesis path of trimetazidine hydrochloride
A technology of trimetazidine hydrochloride and its synthesis method, which is applied in the field of synthesis of anti-anginal drug trimetazidine hydrochloride, can solve problems such as nickel activation and post-processing trouble, difficult preparation of 2-piperazinone, unfavorable industrial production, etc. Achieve the effect of low cost, easy industrialization, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation method 1 of compound III
[0034] Add 12.6g (0.1mol) of 1.2.3-hydroxybenzene (compound II) into a 100ml single-necked bottle, then add 50ml of ethanol, cool to 10°C in an ice-water bath and stir to dissolve. After dissolving, add 2.2g (0.05mol) of paraformaldehyde at 0-5°C, then add 9.4g (0.05mol) of Boc-piperazine in 20ml of ethanol dropwise into the reaction system, and heat up to room temperature for 1.5-2 hours. After the reaction, the reaction solution was poured into 300ml of purified water, and a white solid was precipitated, which was filtered and dried to obtain 12.8g of compound III as a solid, with a yield of 80%.
[0035]
Embodiment 2
[0036] Embodiment 2: The preparation method 2 of compound III
[0037] Add 12.6g (0.1mol) of 1.2.3-hydroxybenzene (compound II) into a 100ml single-necked bottle, then add 50ml of methanol and cool to 10°C in an ice-water bath and stir to dissolve. After dissolving, add 6.0g of formaldehyde (0.05mol) saturated solution dropwise at 0-5°C, then add 9.4g (0.05mol) of Boc-piperazine in 20ml of methanol-alcohol solution dropwise into the reaction system, heat up to room temperature and react for 1.5-2 hours. After the reaction, the reaction solution was poured into 300ml of purified water, and a white solid was precipitated, which was filtered and dried to obtain 12.5 g of compound III as a solid, with a yield of 77%.
Embodiment 3
[0038] Embodiment 3: the preparation of compound IV
[0039] Add 16.2g (0.05mol) of compound III and 37.8g (0.3mol) of dimethyl sulfate into a 250ml three-necked flask, and slowly add aqueous sodium hydroxide solution (12g (0.3mol) of sodium hydroxide, 70g of water), the dropwise addition was completed, and the temperature was raised to 60-70°C and stirred for 2-6 hours. After the reaction was completed, the reaction solution was poured into 300ml of water, filtered by suction, and dried to obtain 16g of compound IV solid, with a yield of 87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com