High performance liquid detection method for content of D-Carnitine in levocarnitine and levocarnitine salt product
A technology of middle-right carnitine and detection method, applied in the field of drug analysis, can solve the problems of complex method, long time-consuming and the like, and achieve the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Choice of Chromatographic Conditions
[0027] 1 Selection of detection wavelength
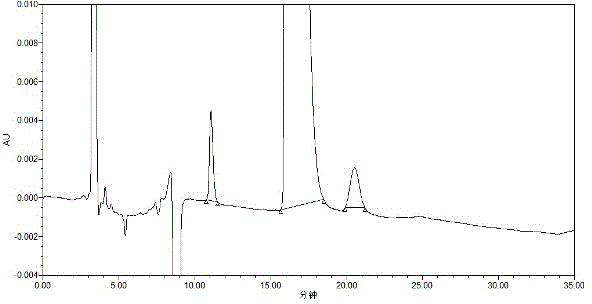
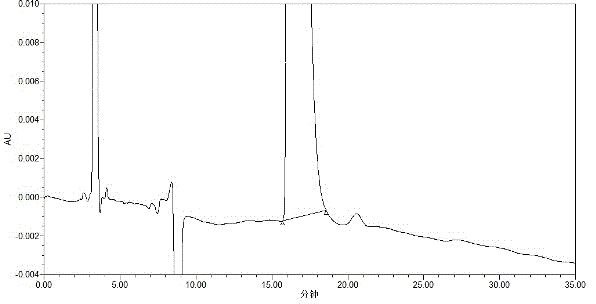
[0028] Take appropriate amount of levocarnitine, dexcarnitine, and levocarnitine impurity A, add water to dissolve and dilute to make solutions with concentrations of 2mg / ml, 2mg / ml and 0.02mg / ml respectively. The UV spectrum shows that levocarnitine, dexcarnitine, and levocarnitine impurity A are end-absorbed. In order to ensure detection sensitivity and system stability, 205nm was selected as the detection wavelength in consideration of the cut-off wavelength of the solvent used.
[0029] 2 Selection of chromatographic column
[0030] Choose from two chiral chromatographic columns, normal phase and reverse phase. When using a normal-phase chiral chromatographic column, the peak time of the three components is very fast and they overlap together and cannot be separated; the β-cyclodextrin and its derivatives in the reverse-phase chiral chromatographic column have the best effect as fi...
Embodiment 2
[0036] Establishment of methodology
[0037] 1 specificity test
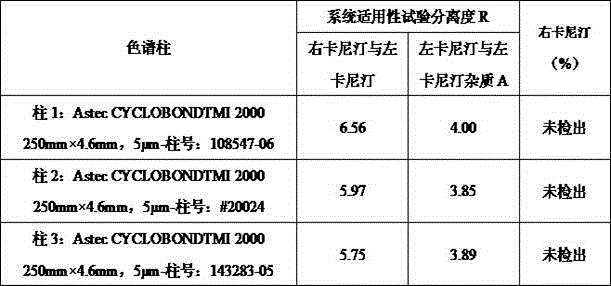
[0038] Precisely measure 10 μl of solvent water and system suitability test solution, inject it into the liquid chromatograph, and record the chromatogram. The degree of separation between impurities A is greater than 1.5.
[0039] 2 Linear range
[0040] Accurately weigh about 0.2114g of levocarnitine reference substance, put it in a 10ml volumetric flask, add water to dissolve and dilute to the mark, shake well, and accurately measure 0.1, 0.25, 0.5, 0.75, 1.0ml of the above solution and place them in five 100ml In a volumetric flask, dilute to the mark with water, shake well, accurately measure 10 μl into the liquid chromatograph, and record the chromatogram. Take the peak area (A) as the ordinate, and the concentration (C) as the abscissa to perform linear regression, and get the regression equation A=403548C-4086.8 r 2 =0.9994 (n=5), the results show that levocarnitine has a good linear relationship in ...
Embodiment 3
[0059] Determination of Dexcarnitine Content in Samples
[0060] 1. Chromatographic conditions:
[0061] Column: Astec CYCLOBOND TM I 2000 chiral column (250mm×4.6 mm, 5μm); detection wavelength: 205nm; mobile phase: triethylamine-acetic acid aqueous solution-acetonitrile (volume ratio 25:75); flow rate: 1.0 ml / min; column temperature: 10 -30°C, injection volume 10μl.
[0062] 2. Preparation of relevant solutions and mobile phases:
[0063] 2.1 Preparation of relevant solutions
[0064] 2.1.1 Preparation of triethylamine-acetic acid aqueous solution:
[0065] Precisely measure 1.5ml of triethylamine reagent, add 900ml of water, adjust the pH to 6.0 with 50% glacial acetic acid aqueous solution, and then add water to 1000ml;
[0066] 2.1.2 Preparation of system suitability solution
[0067] Accurately weigh 0.2056g of levocarnitine reference substance and 2.45mg of dexcarnitine reference substance, and put them in the same 10ml volumetric flask; take another 2.31mg o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com