Preparation method of levocarnitine
A technology of recrystallization and intermediates, which is applied in the field of preparation of levocarnitine, can solve problems such as the inability to racemize enantiomers, and achieve the effect of high optical purity and high yield
Active Publication Date: 2017-05-31
WUXI FORTUNE PHARMA
View PDF2 Cites 7 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0007] The purpose of the present invention is to disclose a preparation method of levocarnitine, which is used to reduce the preparation cost, improve the optical purity of the product, and overcome the defect that the generated enantiomer cannot be racemized in the existing preparation method
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
[0023] The present invention will be described in detail below in conjunction with various embodiments, but it should be noted that these embodiments are not limitations of the present invention, and those of ordinary skill in the art can make functional, method, or structural equivalent transformations or replacements based on these embodiments. , all fall within the protection scope of the present invention.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
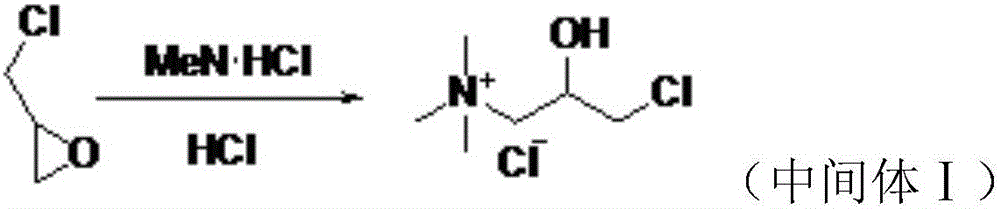
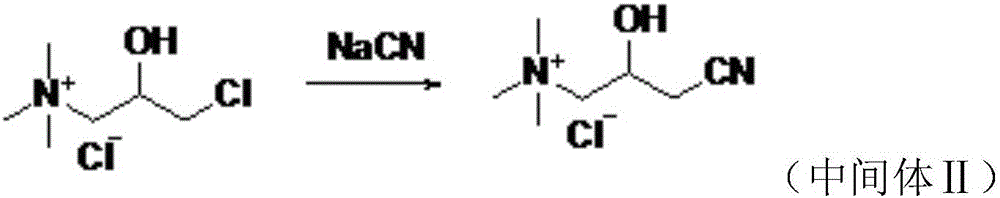
The invention provides a preparation method of levocarnitine. The preparation method comprises the following steps: taking epoxy chloropropane as a starting material, then carrying out amination, cyaniding and carrying out ester exchange under the action of lipase CALB to obtain corresponding chiral ester, then carrying out alkaline hydrolysis and acidification, and then removing chlorine ions under the action of strongly alkaline resin, so that the levocarnitine finished product is obtained. In the invention, acid resin is used in an ester exchange process, and recemization can be realized, so that yield and optical purity of the levocarnitine are improved.
Description
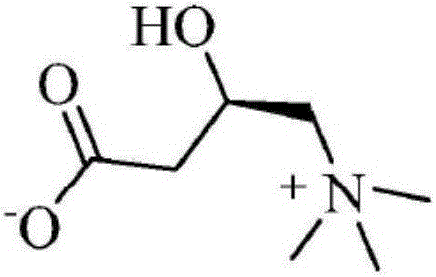
technical field [0001] The invention relates to the field of medicinal chemistry, in particular to a preparation method of levocarnitine. Background technique [0002] Levocarnitine, also known as L-carnitine, vitamin BT, chemical name: chemical name (R)-3-carboxy-2-hydroxy-N,N,N-trimethylpropylammonium hydroxide inner salt, is In vivo natural substance essential in mammalian energy metabolism. The chemical structural formula of L-carnitine is as follows: [0003] [0004] Levocarnitine is the only carrier for fatty acids to be transported to the mitochondria of cells. Therefore, the most important function of L-carnitine is to promote the β-oxidation of fatty acids, reduce the content of serum cholesterol and triglycerides, improve the body's tolerance, and can be used for treatment Chronic renal failure, cardiomyopathy, coronary heart disease, etc. It can also be used as an adjuvant drug for chronic liver disease, and has a certain protective effect on endotoxemia and...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07C227/18C07C229/22
CPCC07C67/08C07C213/04C07C227/18C07C227/26C07C253/14C07C253/30C07C229/22C07C215/08C07C255/26C07C69/63
Inventor 王庆林王涛孙益林王彬彬游本加蒲建新李晓明蒋逸云
Owner WUXI FORTUNE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com