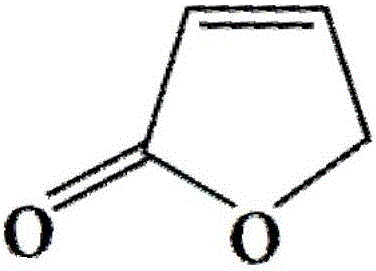
Levocarnitine composition and preparation method thereof
A technology for composition and water for injection, applied in the field of medicine, can solve problems such as poor thermal stability, and achieve the effects of reducing content, improving quality and drug safety, and improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
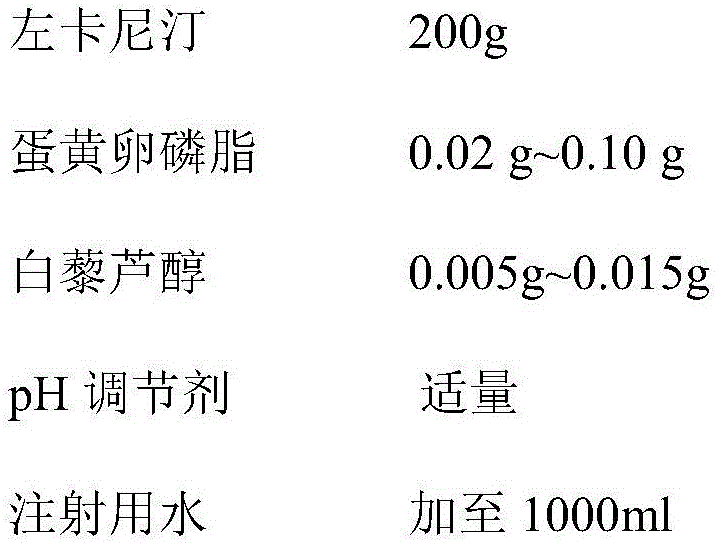
[0049] prescription:
[0050]
[0051]
[0052] Preparation Process:
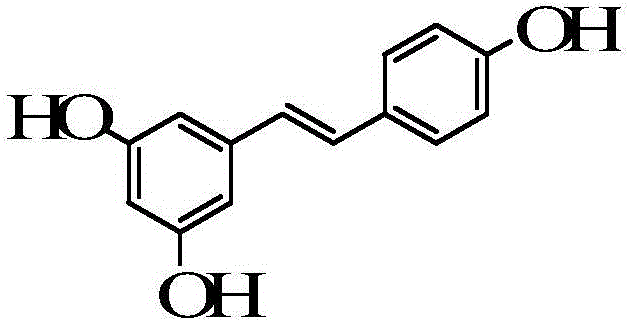
[0053] 1) Weigh 0.005g of resveratrol, add 750ml of water for injection at 38°C to dissolve completely, then add 0.02g of egg yolk lecithin, stir to dissolve, and finally add 200g of levocarnitine to dissolve completely;
[0054] 2) Add an appropriate amount of pH regulator, adjust the pH to 4.8, and continue to inject water to the full amount;
[0055] 3) Add activated carbon for injection to decolorize, filter until clear, filter with a filter membrane, and pot it under nitrogen flow, and finally sterilize it in circulating steam at 100°C for 10 minutes.
Embodiment 2
[0057] prescription:
[0058]
[0059] Preparation Process:
[0060] 1) Weigh 0.01g of resveratrol, add 750ml of water for injection at 39°C to completely dissolve, then add 0.04g of egg yolk lecithin, stir to dissolve, and finally add 200g of levocarnitine to completely dissolve;
[0061] 2) Add an appropriate amount of pH regulator, adjust the pH to 5.0, and continue to inject water to the full amount;
[0062] 3) Add activated carbon for injection to decolorize, filter until clear, filter with a filter membrane, pot it under nitrogen flow, and finally sterilize it in circulating steam at 100°C for 15 minutes.
Embodiment 3
[0064] prescription:
[0065]
[0066] Preparation Process:
[0067] 1) Weigh 0.008g of resveratrol, add 750ml of water for injection at 40°C to dissolve completely, then add 0.04g of egg yolk lecithin, stir to dissolve, and finally add 200g of levocarnitine to dissolve completely;
[0068] 2) Add an appropriate amount of pH regulator, adjust the pH to 5.2, and continue to inject water to the full amount;
[0069] 3) Add activated carbon for injection to decolorize, filter until clear, filter with a filter membrane, fill and seal under nitrogen flow, and finally sterilize in circulating steam at 100°C for 20 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com