Pharmaceutical composition containing L-carnitine and coenzyme Q10 as well as its preparation method
A composition and medicine technology, applied in compound preparation and preparation thereof, pharmaceutical composition comprising L-carnitine and coenzyme Q10 and preparation field thereof, can solve the problem that it cannot be safely used for human injection, large toxic and side effects, and turbid solution and other problems, to achieve good application prospects, good stability, economical and practical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Selection of Solubilizers in Compound Levocarnitine and Coenzyme Q10
[0038] Formulations were prepared according to the ingredients contained in the unit dosage forms shown in Table 1.
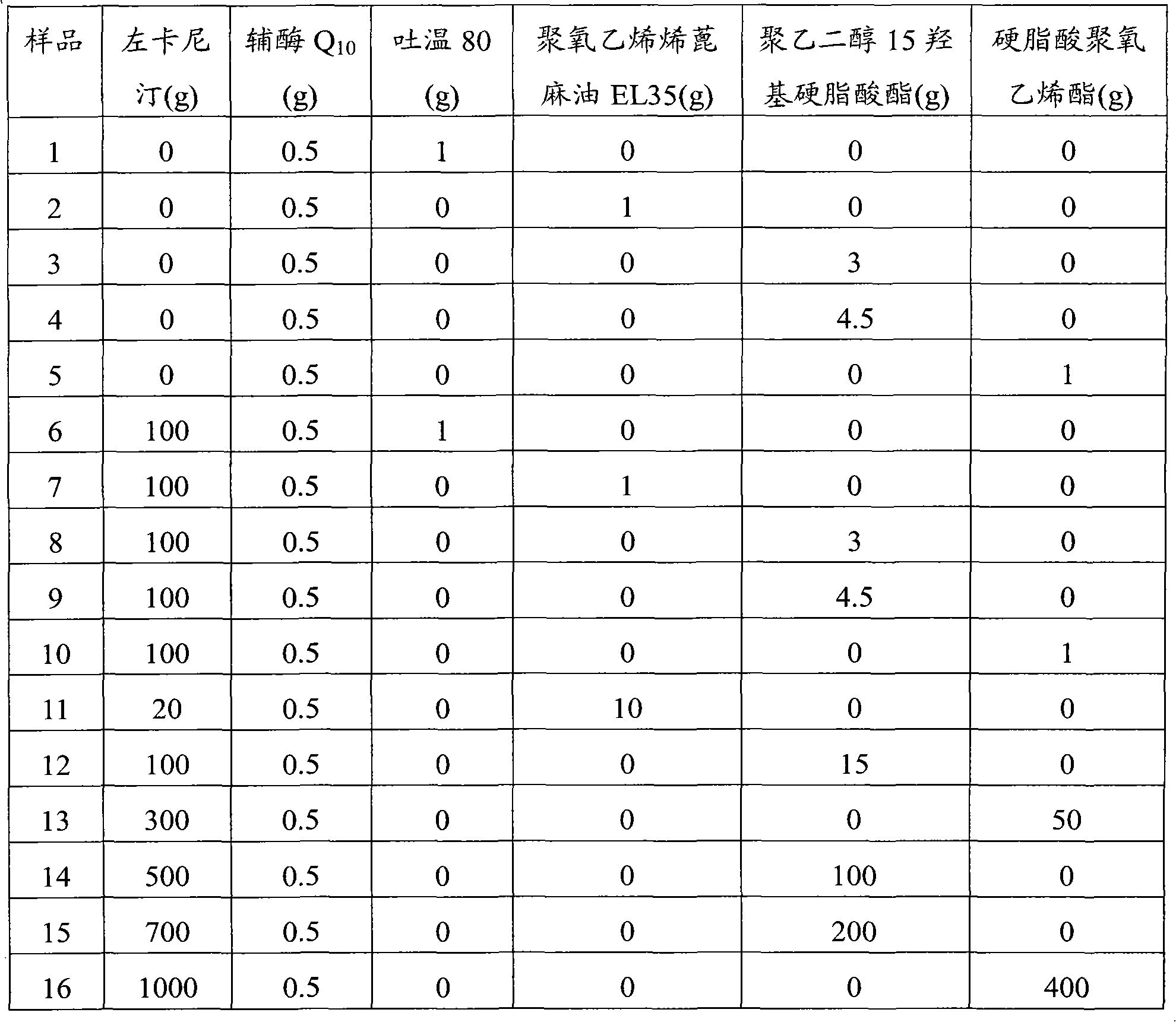
[0039] Table 1 Prescription design
[0040]
[0041] Preparation process of samples 1 to 5: heat the solubilizer to 50-60°C, add coenzyme Q 10 Dissolve in a solubilizer, adjust the pH value to 5.0 with hydrochloric acid, add activated carbon, decarbonize after stirring, add water for injection to make up to 500ml, filter, fill, sterilize, and observe the properties of the liquid at 40°C.
[0042] Preparation process of samples 6 to 16: heat the solubilizer to 50-60°C, add coenzyme Q 10 Dissolve in solubilizer, add levocarnitine after dissolving in water, adjust the pH value to 5.0 with hydrochloric acid, add activated carbon, decarbonize after stirring, add water for injection to 500ml, filter, fill, sterilize, and Place it at 40°C to observe the properties of the liq...
Embodiment 2
[0049] Example 2 Levocarnitine and coenzyme Q 10 oral liquid
[0050] prescription:
[0051]
[0052]
[0053] Preparation:
[0054] Take the prescribed amount of polyoxyethylene castor oil EL35, heat to 30-40°C, add coenzyme Q 10 , stir to dissolve;
[0055] Take 70% of the prescribed amount of purified water, add the prescribed amount of aspartame and stir to dissolve it, add the prescribed amount of levocarnitine and stir to dissolve it;
[0056] Mix the above two medicinal solutions, add malic acid to adjust the pH to 4.0, add 0.5% activated carbon in the total volume, stir evenly, after 30 minutes, decarbonize, add purified water to the total volume, fine filter, fill, and sterilize, that is have to.
Embodiment 3
[0057] Example 3 Levocarnitine and Coenzyme Q 10 Injection
[0058]
[0059] Preparation:
[0060] Take the prescribed amount of polyethylene glycol 15 hydroxystearate, heat to 50-60°C, add coenzyme Q 10 , stir to dissolve;
[0061] In addition, take 70% of the prescribed amount of water for injection, add the prescribed amount of levocarnitine and stir to dissolve it;
[0062] Mix the above two medicinal solutions, add hydrochloric acid to adjust the pH to 4.5, add 0.5% activated carbon in the total volume, stir evenly, after 30 minutes, decarbonize, add water for injection to the total volume, fine filter, fill, seal, sterilize, Instantly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com