Polycrystal substance of acetyl chloride levocarnitine
A technology for acetyl chloride and drugs, which is applied in the field of acetyl levocarnitine chloride crystal form and its preparation, and can solve the problems of high processing cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
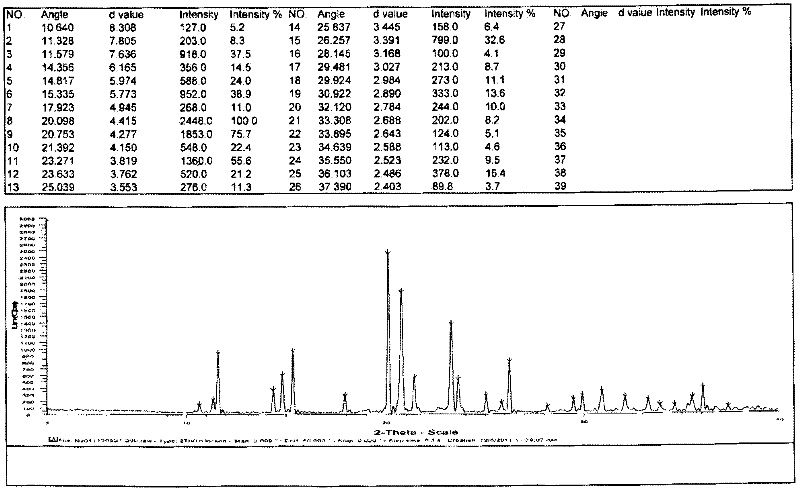
[0022] The preparation of embodiment 1 type I chloride acetyl levocarnitine
[0023] In a 2L reaction flask, add 500mL of glacial acetic acid and 250g of levocarnitine respectively, stir and dissolve at about 50-60°C, and add 215g of acetyl chloride dropwise at a temperature of 50-60°C. After dripping, keep warm for 2-3 hours. After the solvent was recovered under reduced pressure, 1250 mL of isopropanol was added. After the addition was complete, the temperature was raised to reflux to dissolve the liquid, and then the temperature was lowered to below 15° C. and stirred for 1 hour for crystallization. Filter, wash with 300 mL of isopropanol, suck dry, and dry in vacuo to obtain 338 g of white crystalline solid. The yield is 90.9%. Purity expressed by HPLC area %: 98.6%, levocarnitine 0.5%, 3-carboxyallyl-N,N,N-trimethylammonium chloride 0.5%, enantiomer 0.2%.
Embodiment 2
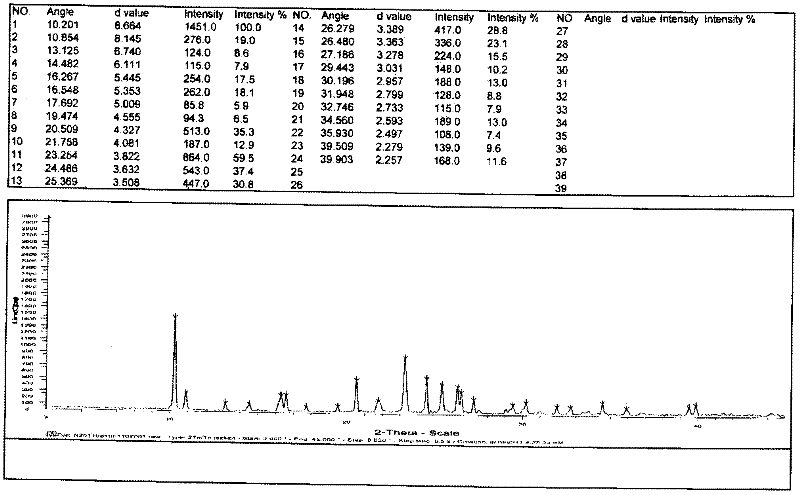
[0024] The preparation of embodiment 2 type II chloride acetyl levocarnitine
[0025] In a 2L reaction flask, add 500mL of glacial acetic acid and 250g of levocarnitine respectively, stir and dissolve at about 50-60°C, and add 215g of acetyl chloride dropwise at a temperature of 50-60°C. After dripping, keep warm for 2-3 hours. After the solvent was recovered under reduced pressure, 500 mL of absolute ethanol was added. After the addition was complete, the temperature was controlled below 15°C and the crystallization was stirred for 1 h. Filter, wash with absolute ethanol, suck dry, and vacuum dry to obtain 320 g of white crystalline solid. Yield 86.1%. Purity expressed by HPLC area %: 99.3%, levocarnitine 0.1%, 3-carboxyallyl-N,N,N-trimethylammonium chloride 0.1%, enantiomer 0.1%. The obtained solid was identified by X-ray powder diffraction as Form II acetyl-levocarnitine chloride.
Embodiment 3
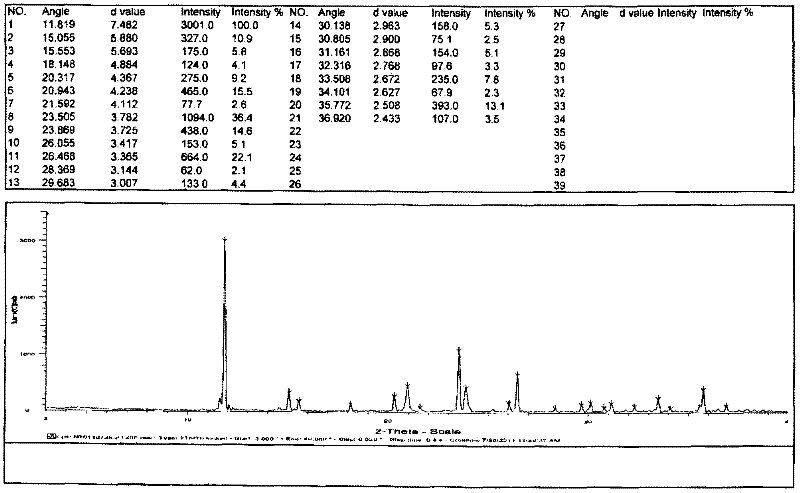
[0026] The preparation of embodiment 3 type III chloride acetyl levocarnitine
[0027] Add 100g of acetyl levocarnitine chloride prepared in Example 1 into 200mL of glacial acetic acid, heat to 50-60°C, stir to dissolve and filter, drop the filtrate into 200mL of acetone, cool to below 10°C and stir to analyze crystallized for 1 h, filtered, washed with acetone, and dried in vacuo to obtain 78 g of white crystalline solid with a yield of 78%. Purity expressed by HPLC area %: 99.4%, levocarnitine 0%, 3-carboxyallyl-N,N,N-trimethylammonium chloride 0%, enantiomer 0%. The obtained solid was identified by X-ray powder diffraction as Form III acetyl-levocarnitine chloride.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com