High-yield synthesis method of trenbolone acetate
A technology for the synthesis of trenbolone acetate and its synthesis method, which is applied in the field of synthesis of trenbolone acetate, and can solve the problems of low yield, many by-products, and low quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
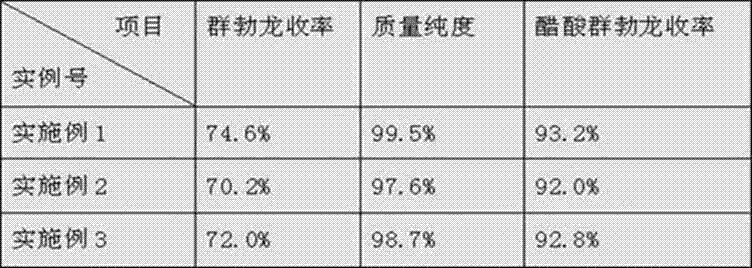
Embodiment 1
[0021] 1. Etherification reaction: Put 80kg of alcohol into a 500L reaction tank and cool down to 0°C, start stirring, add 10kg of acetyl chloride dropwise, and control the temperature at 0-10°C. After the dropwise addition, 10 kg of estro-4,9-diene-3,17-dione was added, and stirred and kept at 10-13° C. for 2 hours. After the heat preservation is completed, the feed liquid is pumped into the sodium carbonate aqueous solution, and the temperature is controlled at 20-25°C. After the dropwise addition, the temperature is controlled at 20-25°C, stirred for 30 minutes, filtered with suction, washed with a large amount of water until neutral, and centrifuged to obtain a wet feed.
[0022] 2. Reductive hydrolysis dehydrogenation reaction: pump 100kg of methanol into a 500L reaction tank, put in the wet material of the previous step, cool down to 0°C, add 5kg of potassium borohydride, control the temperature at 0-5°C when adding, and stir after the addition is complete Keep warm for ...
Embodiment 2
[0026] Embodiment 2: The raw materials involved in the etherification reaction and the consumption were changed to 75 kg of methanol, 5 kg of acetyl chloride, and 5 kg of estro-4,9-diene-3,17-dione;
[0027] The raw materials and dosage involved in the reduction reaction are changed to 95 kg of methanol, 95 kg of wet reaction material, 4 kg of potassium borohydride, and 180 kg of dichloromethane;
[0028] The raw materials and dosage involved in the hydrolysis reaction are changed to 180kg of oil, 65kg of methanol, 18kg of dichloromethane, and 6kg of anhydrous magnesium sulfate;
[0029] The raw materials involved in the dehydrogenation reaction and the consumption are changed to 180kg of dichloromethane, 180kg of filter cake, and 6kg of DDQ;
[0030] The raw materials involved in the acylation reaction and the amount used were changed to 95 kg of benzene, 6 kg of pyridine, 6 kg of trenbolone, 0.3 kg of 4-DMAP, 6 kg of acetyl chloride, 6 kg of ethyl acetate, and 20 kg of petro...
Embodiment 3
[0032] Example 3: The raw materials and dosage involved in the etherification reaction were changed to 85 kg of methanol, 15 kg of acetyl chloride, and 15 kg of estro-4,9-diene-3,17-dione;
[0033] The raw materials and dosage involved in the reduction reaction are changed to 105kg of methanol: 105kg of wet reaction material, 10kg of potassium borohydride, and 210kg of dichloromethane;
[0034] The raw materials involved in the hydrolysis reaction and the consumption are changed to 210kg of oil, methanol, 75kg, 210kg of dichloromethane, and 15kg of anhydrous magnesium sulfate;
[0035] The raw materials involved in the dehydrogenation reaction and the consumption are changed to 210kg of dichloromethane, 210kg of filter cake, and 12kg of DDQ;
[0036] The raw materials involved in the described acylation reaction and the consumption are changed to 105 kg of benzene, 12 kg of pyridine, 12 kg of trenbolone, 0.6 kg of 4-DMAP, 12 kg of acetyl chloride, 12 kg of heptanoyl chloride, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com