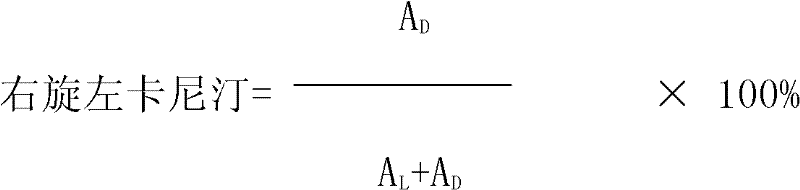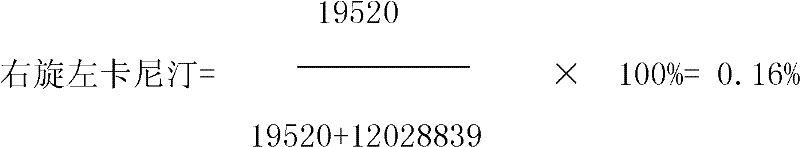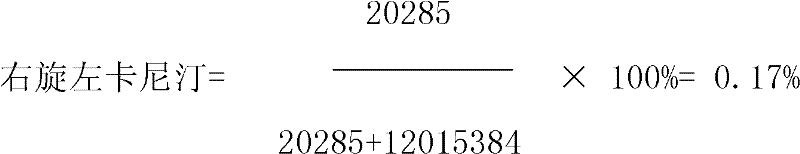Detection method for dextroisomer in levocarnitine
A detection method and isomer technology, applied in the field of drug analysis, can solve problems such as no related reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The following examples will help to understand the present invention, but the examples are only to illustrate the present invention, and the present invention is not limited to these contents.
[0025] 1. Chromatographic conditions:
[0026] Chromatographic column: C18 column filled with octadecylsilane bonded silica gel, particle size 5μm; detection wavelength: excitation wavelength λex=260nm, fluorescence wavelength λem=315nm; mobile phase: tetra-n-butylammonium hydroxide-methanol and acetonitrile - Water; column temperature: 30°C, injection volume 5 μl.
[0027] 2. Preparation of relevant solutions and mobile phases:
[0028] 2.1 Preparation of relevant solutions
[0029] 2.1.1 Preparation of tetra-n-butylammonium hydroxide solvent:
[0030] Accurately draw 24.4ml of 55% tetra-n-butylammonium hydroxide reagent, dilute to 1000ml with water, shake well, and adjust the pH to 7.0 with phosphoric acid.
[0031] 2.1.2 Preparation of D-Fremoxycarbonyl Chloride Reagent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com