Levocarnitine coating tablet and preparation method thereof
A technology for coating tablets and film coating, which can be applied to pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve problems such as hygroscopicity, and achieve low production cost, high efficiency, Simple to use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
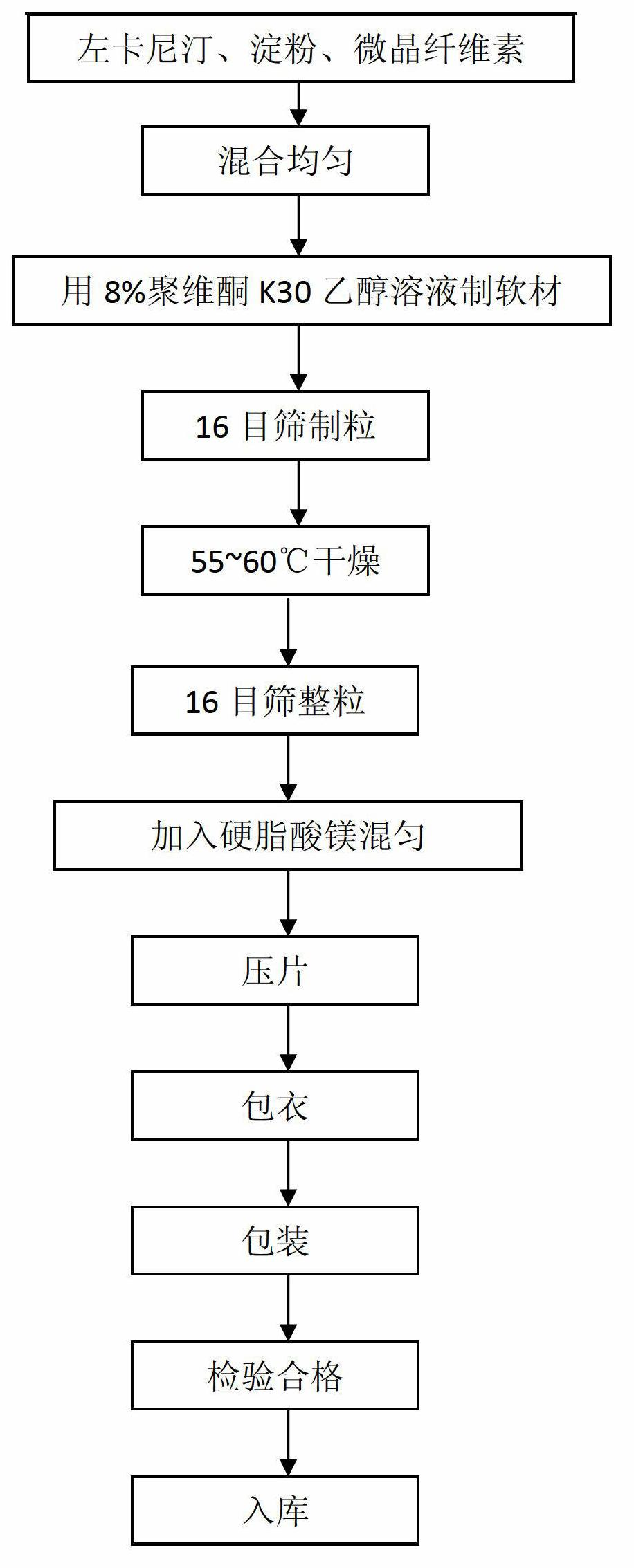
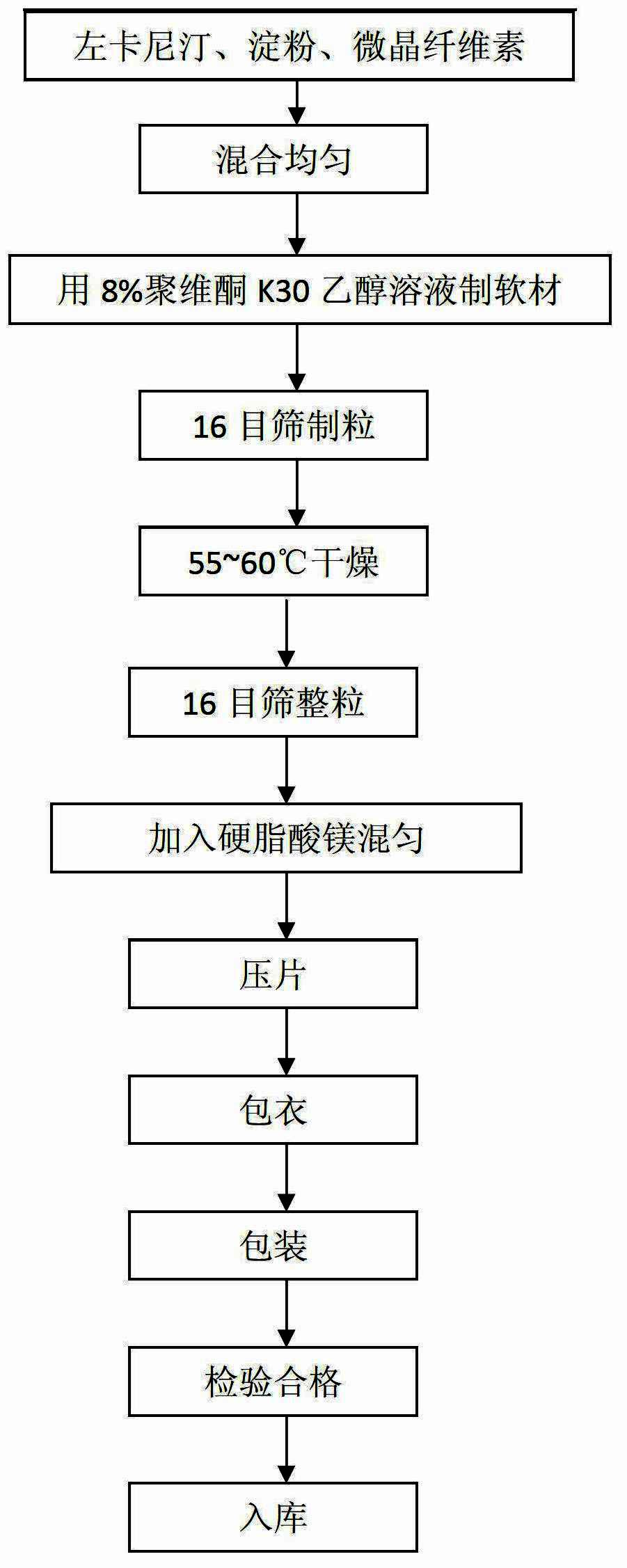
[0020] The present embodiment prepares 1000 levocarnitine coated tablets, the steps are as follows:
[0021] (1) Pass levocarnitine, starch, microcrystalline cellulose and magnesium stearate through 80-mesh sieve respectively, and set aside;
[0022] (2) Take standby levocarnitine 250g, starch 75g and microcrystalline cellulose 121.5g, mix and make mixed powder;
[0023] (3) Add the ethanol solution of povidone K30 of 15g8% mass percent concentration in the mixed powder of levocarnitine, starch and microcrystalline cellulose to make soft material and cross 16 mesh sieves and make granule, at 55-60 Dry at ℃ temperature;
[0024] (4) Add 3.5 g of magnesium stearate to the dried granules and cross a 16-mesh sieve and mix uniformly;
[0025] (5) compressing the uniformly mixed granules to make levocarnitine tablet core;
[0026] (6) Put the levocarnitine tablet cores into the coating pan to coat the pressed levocarnitine tablet cores, adjust the speed of the coating pan to 6 rp...
Embodiment 2
[0029] The present embodiment prepares 1000 levocarnitine coated tablets, the steps are as follows:
[0030] (1) Pass levocarnitine, starch, microcrystalline cellulose and magnesium stearate through 80-mesh sieve respectively, and set aside;
[0031] (2) Take standby levocarnitine 230g, starch 55g and microcrystalline cellulose 100g, mix and make mixed powder;
[0032] (3) Add the ethanol solution of povidone K30 of 10g8% mass percentage concentration in the mixed powder of levocarnitine, starch and microcrystalline cellulose to make soft material and cross 16 mesh sieves and make granule, at 55-60 Dry at ℃ temperature;
[0033] (4) Add 1.5 g of magnesium stearate to the dried granules and cross a 16-mesh sieve to mix evenly;
[0034] (5) compressing the uniformly mixed granules to make levocarnitine tablet core;
[0035] (6) Put the levocarnitine tablet cores into the coating pan to coat the pressed levocarnitine tablet cores, adjust the speed of the coating pan to 6 rpm, ...
Embodiment 3
[0038] The present embodiment prepares 1000 levocarnitine coated tablets, the steps are as follows:
[0039] (1) Pass levocarnitine, starch, microcrystalline cellulose and magnesium stearate through 80-mesh sieve respectively, and set aside;
[0040] (2) Take standby levocarnitine 270g, starch 95g and microcrystalline cellulose 140g, mix and make mixed powder;
[0041] (3) Add the ethanol solution of povidone K30 of 20g8% mass percentage concentration in the mixed powder of levocarnitine, starch and microcrystalline cellulose to make soft material and cross 16 mesh sieves and make granule, at 55-60 Dry at ℃ temperature;
[0042] (4) Add 5.5 g of magnesium stearate to the dried granules and cross a 16-mesh sieve to mix evenly;
[0043] (5) compressing the uniformly mixed granules to make levocarnitine tablet core;
[0044] (6) Put the levocarnitine tablet cores into the coating pan to coat the pressed levocarnitine tablet cores, adjust the speed of the coating pan to 6 rpm, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com