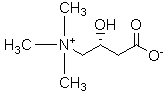
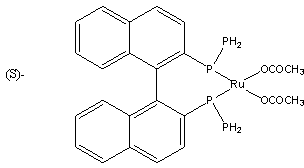
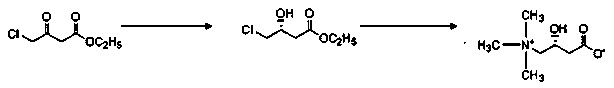Preparation method of L-carnitine compound
A technology of compounds and catalysts, applied in the field of preparation of levocarnitine compounds, which can solve the problems of many side reactions, no industrial production of chiral catalysts, and high temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 1. Selectivity test of reaction temperature in step (1)
[0018] Table 1 Reaction temperature selectivity test
[0019] Reaction temperature (hydrogen pressure 6MPa) 25℃ 40℃ 50℃ 60℃ 80℃ Reaction time There are still some raw materials when the reaction time exceeds 6 hours There is still a small amount of raw materials when the reaction time exceeds 4 hours 3 hours 1.5 hours 0.5 hours
[0020] The results show that the effect is the best when the reaction temperature of step (1) is 50-80°C.
[0021] 2, Mole ratio selectivity test of ethyl 4-chloroacetoacetate and catalyst
[0022] Table 2 Molar ratio selectivity test of ethyl 4-chloroacetoacetate and catalyst
[0023] The molar ratio of substrate to catalyst 1:50 1:100 1:1000 1:10000 1:20000 Optical purity 99%e.e. 99%e.e. 98% e.e. 98% e.e. 95%e.e.
[0024] The results showed that when the molar ratio of ethyl 4-chloroacetoacetate to catalyst was 1:50...
Embodiment 2
[0029] Example 2 Preparation of (R)-4-chloro-3-hydroxybutyric acid ethyl ester
[0030] In 0.5L hydrogenation autoclave, add 4-chloroacetoacetate ethyl ester 100g, catalyst Ru(OCOMe) 2 [(S)-BINAP] 0.1g and 88g ethanol, seal the reactor, replace the air in the reactor with hydrogen for 3 times, keep the pressure of the reactor at 7Mpa, raise the temperature to 70°C, stir for 1 hour, and then cool down to room temperature. The reaction solution was concentrated under reduced pressure, and the remaining brown oil was distilled under high vacuum to obtain 95 g of (R)-ethyl 4-chloro-3-hydroxybutyrate as a colorless transparent liquid, with a yield of 93.9% and an optical purity of 98% e.e.
Embodiment 3
[0031] Example 3 Preparation of Levocarnitine Compounds
[0032] Add 250g of purified water into a 1000mL four-neck flask, add 13.5g of sodium hydroxide, and stir until the solids are completely dissolved. The temperature was lowered to -5°C, and 65 g of 33% trimethylamine aqueous solution was added dropwise to control the temperature to -5°C. After the dropwise addition, 35 g of (R)-ethyl 4-chloro-3-hydroxybutyrate was added dropwise, and the temperature was controlled at -5°C. After the dropwise addition, the reaction was continued at -5°C for 1 hour, and then the temperature was raised to room temperature for 12 hours. Concentrated hydrochloric acid was added dropwise to adjust the pH value to 6, and purified by cationic resin column.
[0033] Add the reaction liquid to control the flow rate under the column to be ≤0.5L / hour. After the feeding is complete, add purified water to the resin column, start to control the flow rate under the column to be ≤0.5L / hour, and end whe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com