Patents
Literature
256results about How to "Non-allergenic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mask liquid with multiple skin care effects and preparation method thereof
InactiveCN106074315AImprove moisturizing abilityAdjust water and oil balanceCosmetic preparationsToilet preparationsLong lastingWrinkle
The invention discloses mask liquid with multiple skin care effects and a preparation method thereof, aims at providing mask liquid which has effects of skin whitening, speckle removing, wrinkle smoothing, inflammation relieving and diminishing, long-lasting moisturizing and grease balancing, and belongs to the technical field of cosmetics. According to the technical scheme, the mask liquid is prepared from, by weight, 0.5%-3% of sericin, 3.011%-14.02% of a plant extract, 1.11%-8.35% of a moisturizer, 8%-15% of polyalcohol, 0.1%-1.5% of active peptides, 0.08%-0.15% of Carbomer, 0.08%-0.15% of triethanolamine, 0.02%-0.06% of disodium EDTA, 0.3%-0.5% of a preservative and the balance deionized water.
Owner:GUANGDONG BAWEI BIOLOGICAL TECH CO LTD
External plaster for treating baby heat cough and preparing method
InactiveCN1586552AHave antitussiveNo toxic performanceAnthropod material medical ingredientsAerosol deliveryMedicineForsythia
The external plaster for treating baby's heat cough is developed based on the pathological features and the pathological cause of baby's heat cough. It is prepared with 17 kinds of Chinese medicinal materials including forsythia, sweet wormwood herb, skullcap root, bitter orange, etc. and through decoction, concentration, crushing, mixing and other steps. The external plaster for treating baby's heat cough has the functions of dispelling wind, clearing away heat, promoting lung-Qi flow, eliminating phlegm, etc. and is used mainly for treating baby's heat cough caused by different reasons. The medicine has convenient use and obvious effect, and the preparation process is suitable for industrial production.
Owner:左耀武
External plaster for treating child fever and preparing method
InactiveCN1899543AEnhance immune functionNo toxic performanceAnthropod material medical ingredientsAntipyreticDiseaseCurative effect
The externally applied plaster for treating infantile fever is developed on the infantile physiological and pathological characteristics and fever cause and is prepared with 18 kinds of Chinese medicinal materials, including sweet wormwood, eucalyptus leaf, mint, schizonepta, etc. and through decoction, concentration, crushing, mix and other steps. It has the functions of dispelling wind and other pathogenic evil, clearing away heat and toxic material, resolving stagnation and alleviating mental depression, is used to treat infantile fever diseases, and has obvious curative effect and high safety.
Owner:赤峰市创新中蒙药研究所有限公司
Moisturizing eye mask prepared from bacterial cellulose
InactiveCN102784071AReliable hardnessGood flexibilityCosmetic preparationsToilet preparationsBiotechnologyMembrane surface
The invention discloses a moisturizing eye mask prepared from bacterial cellulose. The method for preparing a moisturizing eye mask from bacterial cellulose comprises: inoculating 1-2 rings of activated inclined seed Gluconacetobacter xylinum into a medium, and conducting oscillating culture to obtain a seed solution; then inoculating the seed solution into the medium, fully oscillating the bacterial solution obtained by mixing the seed solution and the medium to make them mixed uniformly, then conducting stationary culture, thus generating a bacterial cellulose membrane floating on the solution surface, flushing the generated bacterial cellulose membrane with water to remove the medium and impurities on the membrane surface, then immersing the membrane in an alkali solution to remove thalli and residual medium from the membrane, and finally flushing the membrane with distilled water till the pH value of the membrane is measured up to 7-7.2, thus obtaining a bacterial cellulose wet membrane, which is then cut into an eye mask. The eye mask has the advantages of good moisturizing property and biological tissue compatibility, no toxicity, harmlessness, and simple material processing technology.
Owner:SHANGHAI INST OF TECH
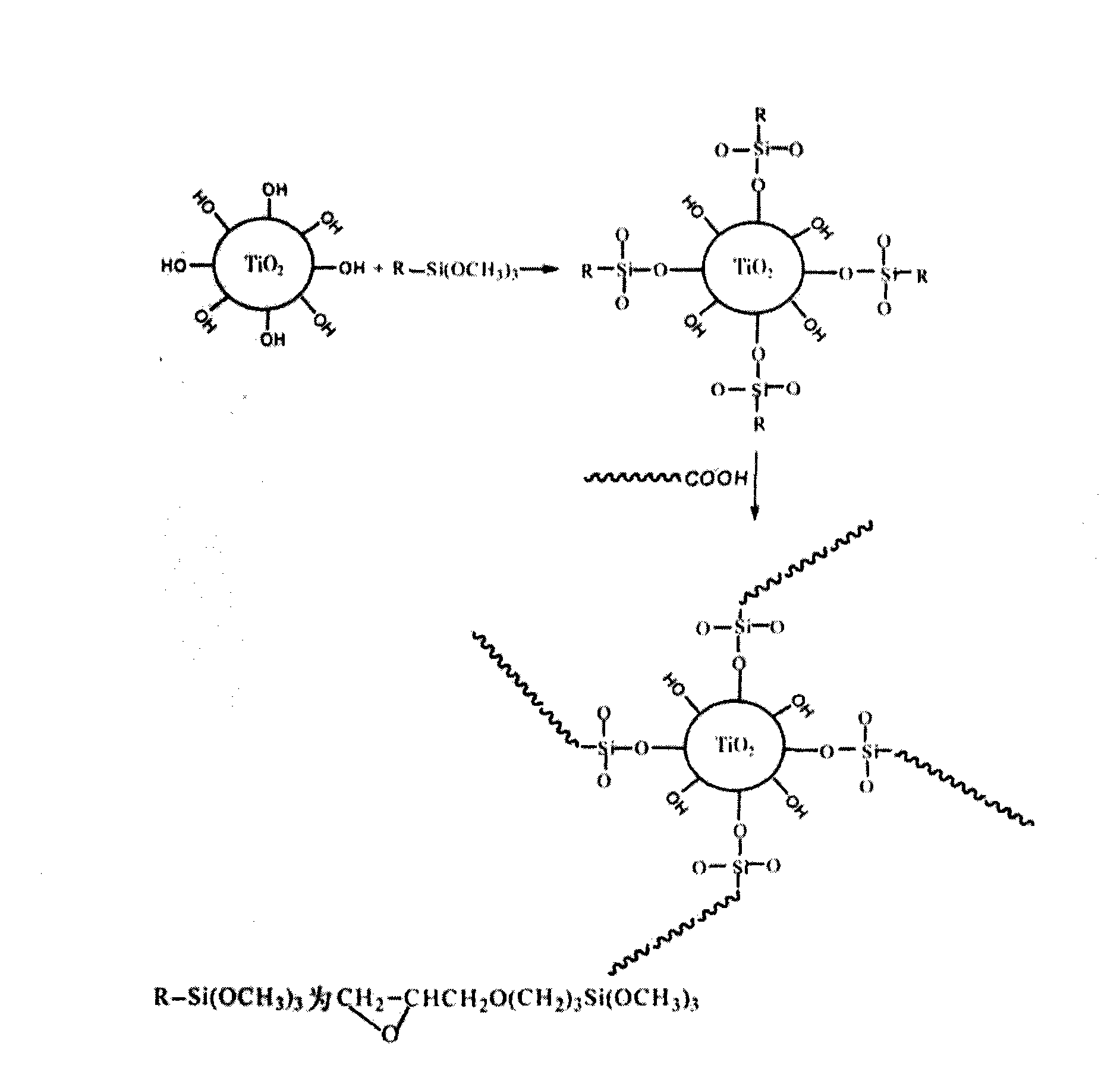
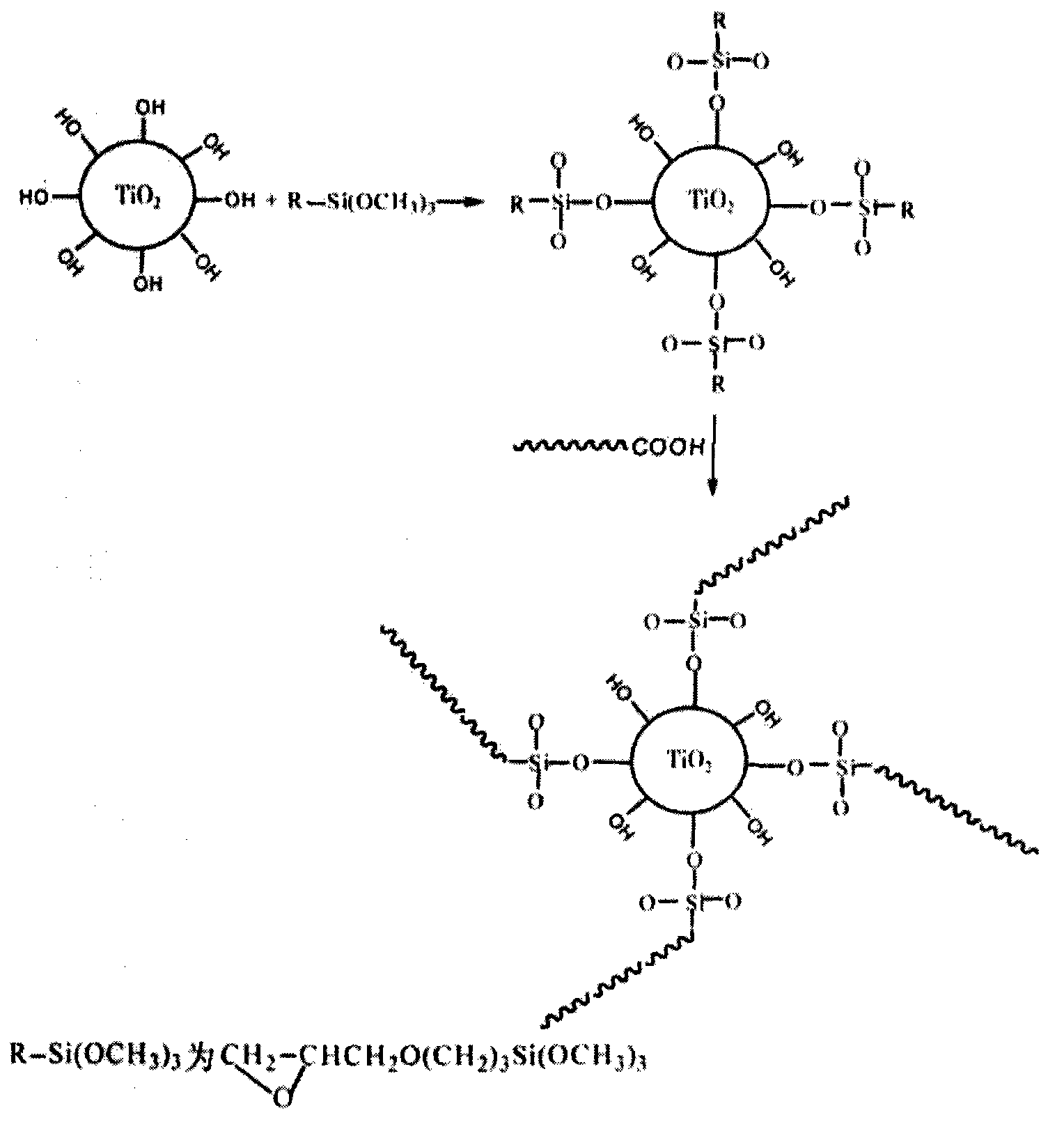
Method for preparing finishing agent of modified nano titanium dioxide coating layer of electromagnetic shielding fabric
InactiveCN101768853AImprove performanceNot easy to reuniteMagnetic/electric field screeningFibre treatmentCross-linkUltraviolet
The invention relates to a method for preparing a finishing agent of a modified nano titanium dioxide coating layer of an electromagnetic shielding fabric. The technical problem of the invention to be solved is that the method for preparing the finishing agent of the modified nano titanium dioxide coating layer of the electromagnetic shielding fabric is provided, so that the electromagnetic shielding fabric has excellent performance of ultraviolet ray resistance, antibacterium, self-cleaning and the like, and the original characteristic of low surface resistance can be maintained at the same time. The technical scheme for solving the problem is that nano TiO2 is modified by a special process, and then the modified nano TiO2 is mixed uniformly with resin and a cross-linking agent according to the proportion, so that the finishing agent of the modified nano titanium dioxide coating layer of the electromagnetic shielding fabric is obtained. The invention can be used for the textile industry of the electromagnetic shielding fabric.
Owner:ZHEJIANG SAINTYEAR ELECTRONICS TECH
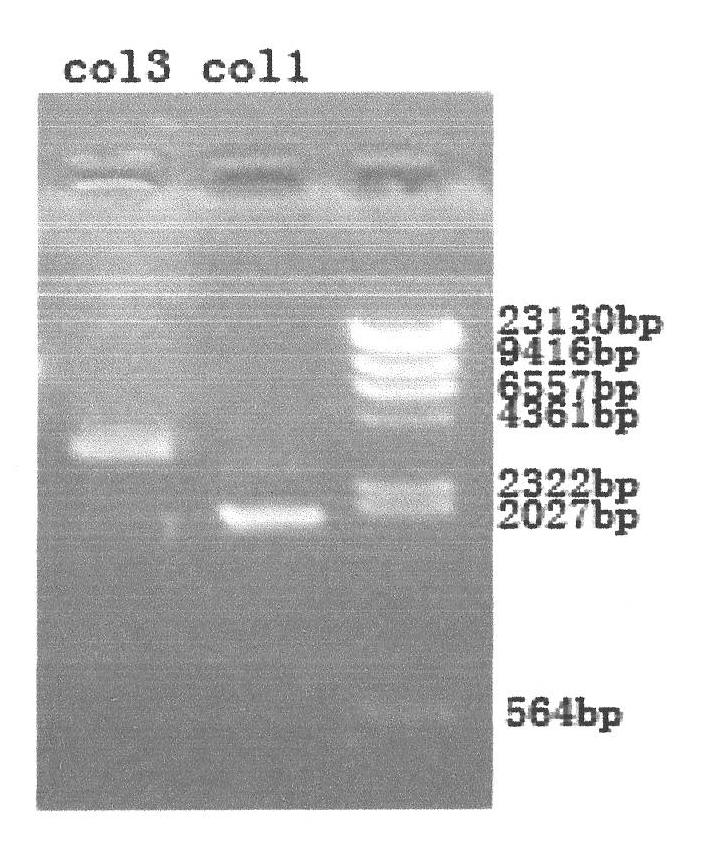
Gene recombination human collagen fusion peptide segment, preparation method and application thereof
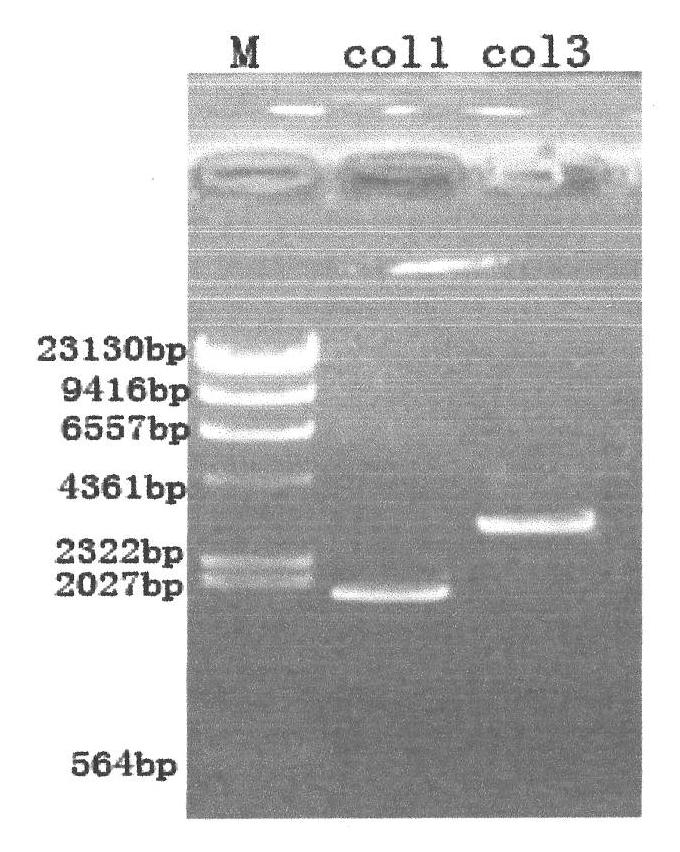
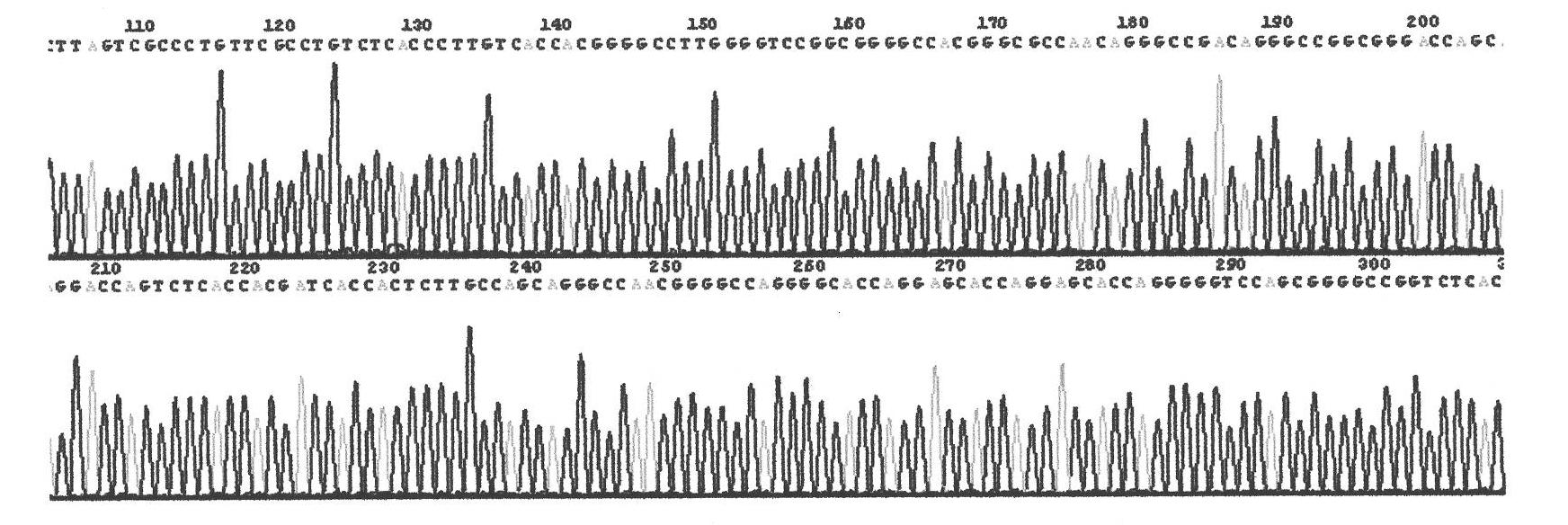
ActiveCN102020716ANon-allergenicGood moisturizing effectCosmetic preparationsFungiGenetic engineeringChemistry
The invention relates to a gene recombination human collagen fusion peptide segment. The overall length of the peptide segment comprises 839 amino acids, the nitrogen end of the peptide segment comprises a type III human collagen peptide section of 908-1137, which totally contains 229 amino acids, and the carbon end of the peptide segment comprises a type I human collagen peptide segment of 497-1104, which total contains 608 amino acids, wherein the two peptide segments are connected by using glutamic acid and phenylalanine. The preparation method of the peptide segment comprises the following steps of: constructing cDNAs of clone type I (COL 1) and type III (COL 3) human collagen genes and a Pichia pastoris recombinant expression vector pPOC9K-COL3a1-COL1a1; constructing Pichia pastoris genetic engineering bacteria SMD1168-COL3a10COL1a1 of the gene recombination human collagen fusion peptide segment; preparing the gene recombination human collagen fusion peptide segment, and the like. Proved by tests, the gene recombination human collagen fusion peptide segment prepared by adopting the method can be used as a material for preparing cosmetics.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
External plaster for treating child cold type cough and preparing method
InactiveCN1899550AEnhance immune functionSolve the problem of needle and drug refusalAerosol deliveryOintment deliveryCurative effectTangerine Peel
The externally applied plaster for treating infantile cold type cough is developed on the infantile physiological and pathological characteristics and cough cause and is prepared with 13 kinds of Chinese medicinal materials, including purple perilla, hogfennel root, tangerine peel, eucalyptus leaf, etc. and through decoction, concentration, crushing, mix and other steps. It has the functions of dispelling wind and cold, opening the inhibited lung energy and relieving cough, regulating qi, etc. and is used to treat infantile cough caused by external wind and cold and dyspepsia due to improper feeding, and has obvious curative effect and high safety.
Owner:赤峰市福善堂医药有限公司
Method for extracting dihydroquercetin form larch
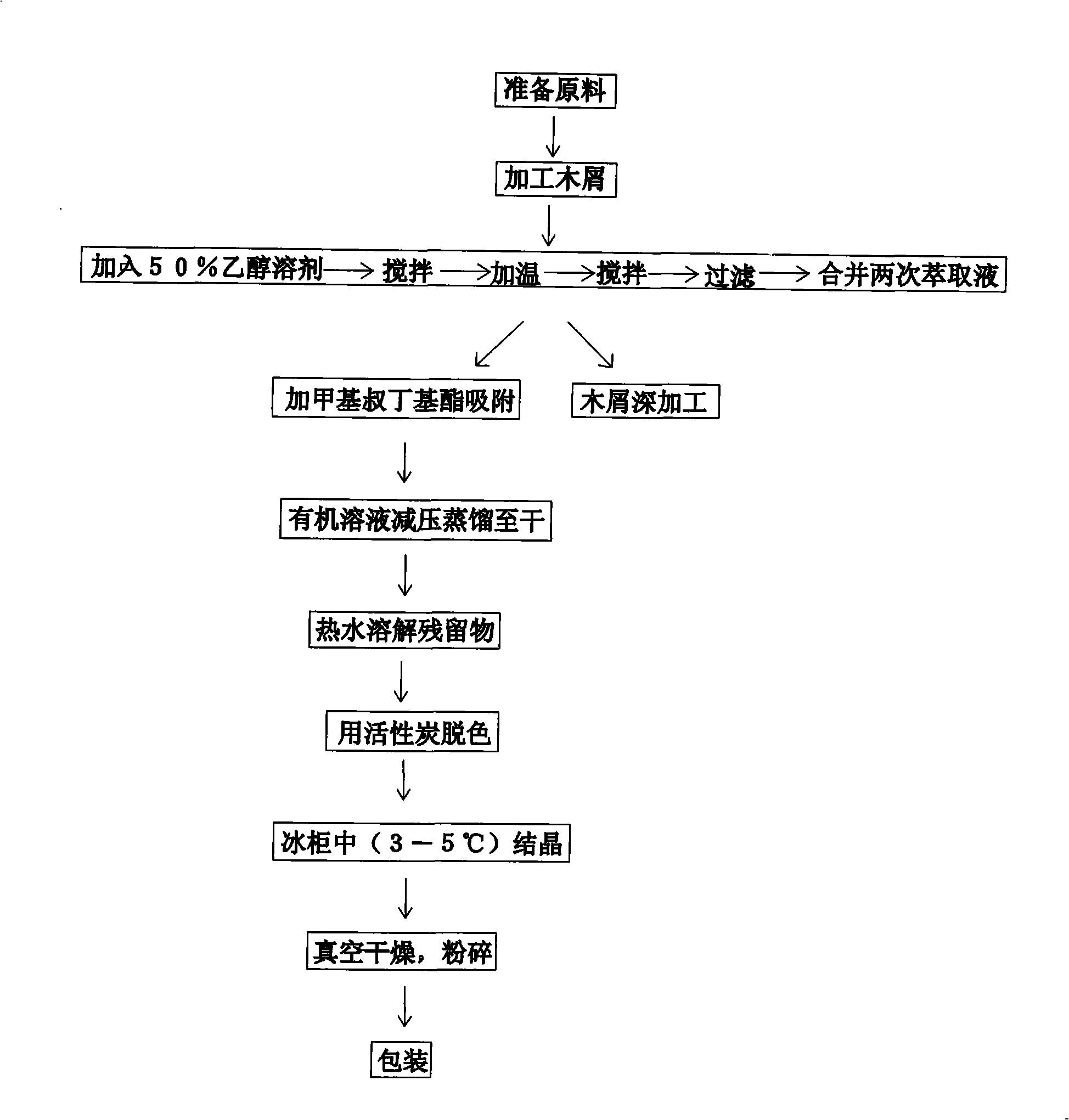
InactiveCN101333203AFree radical scavengingLesion hindranceOrganic chemistryConiferophyta medical ingredientsLarchUnit/Kilogram
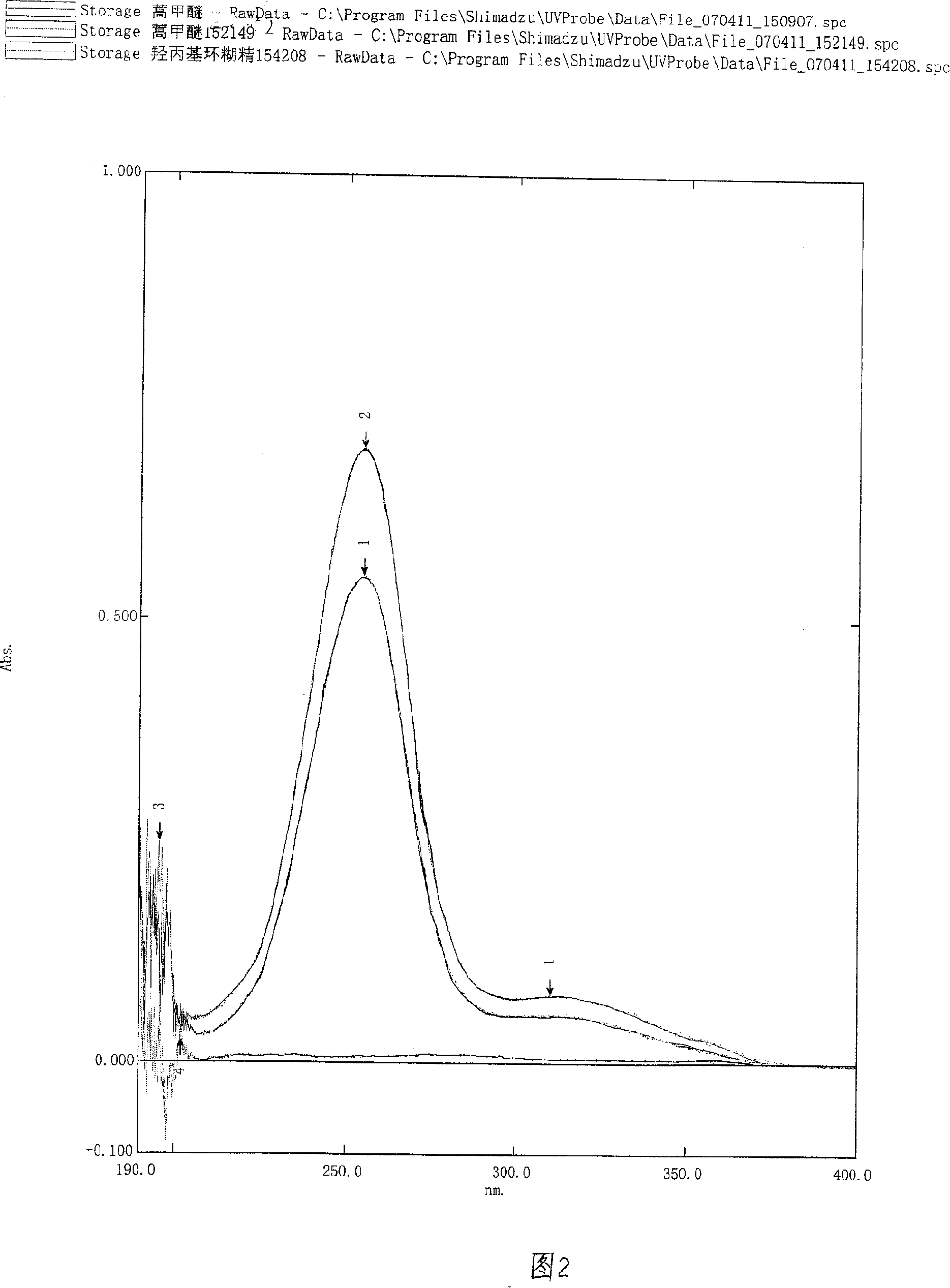
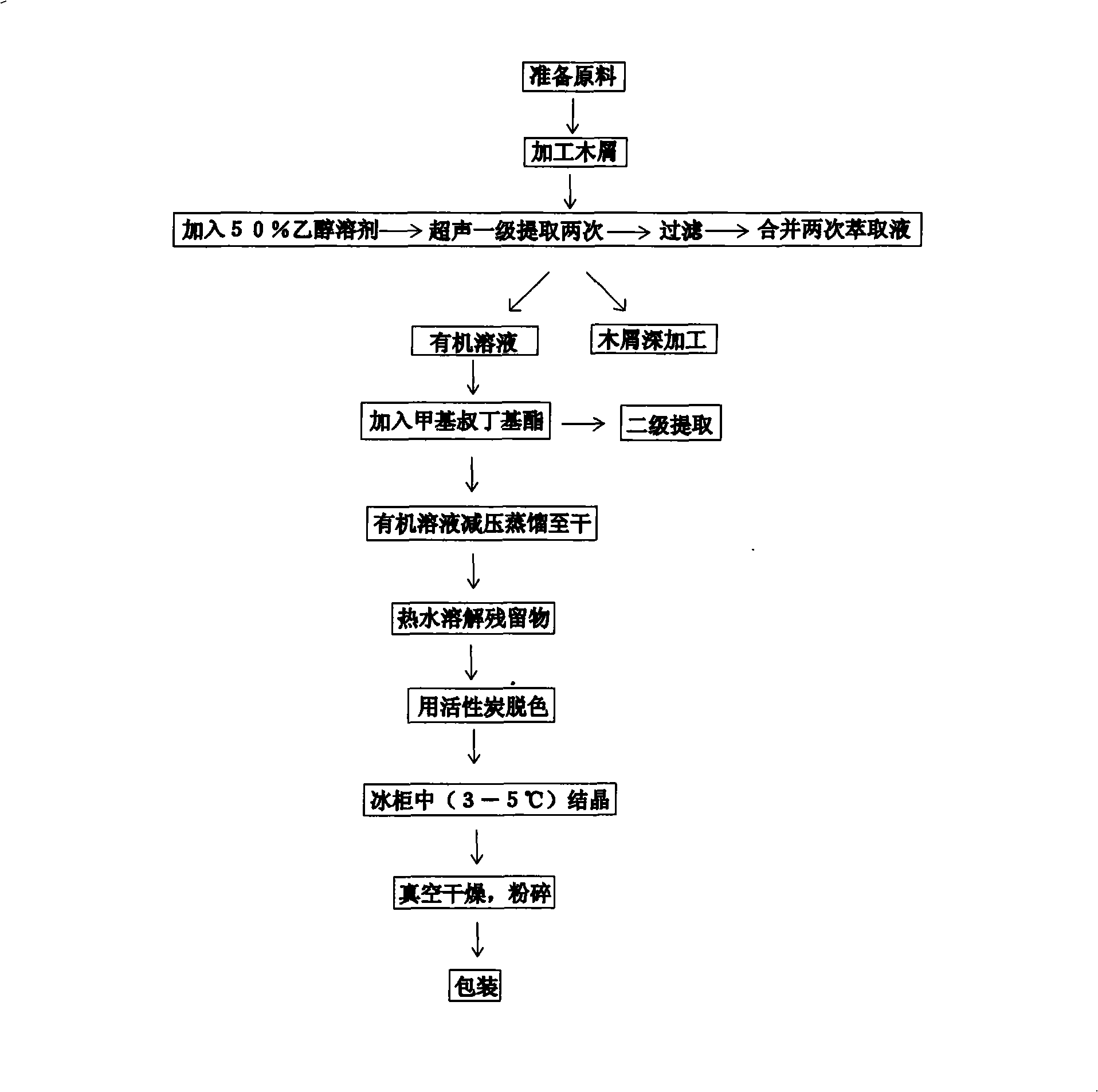
Disclosed is a method for extracting dihydro quercetin from larch. The invention is an improved production method. Due to different production materials and extraction processes, the dihydro quercetin products in U.S. and European markets are very expensive, at a price of 200-1000 euros per gram. Such a high market price economically limits the wide range of practicability of dihydro quercetin products. During the pretreatment of raw materials, 50 kilograms of larch wood chips and 500-800 liters of 50% ethanol solution are added into a reactor to get fully stirred and mixed; then the mixture is extracted and filtered for the second extraction; the filtrates are combined and adsorbed; the organic solvents are collected and dried by a vacuum evaporator through organic phase vacuum distillation; according to a weight proportion of 10:1, the residues are dissolved with hot water, decolorized with activated carbon and then crystallized at a temperature of 3-5 DEG C to precipitate the dihydro quercetin product which is then crushed after vacuum drying to finished product. The invention is used to extract dihydro quercetin from larch.
Owner:黑龙江花旗科技发展有限公司 +2
Method of modifying lactoalbumin by enzymatic method and its application
ActiveCN1596676AImprove the degree of enzymatic hydrolysisHigh selectivityMilk preparationEnzyme methodProtein formation
A process for modifying the lactoprotein by enzyme method features that the immobilized composite enzyme, the ionically regulatory protein structure and heat treating technique are used as selectively hydrolyze the alpha S-casein and beta-lactoglobulin in lactoprotein, resulting in easy digestion and low sensitization. It can be used for composite milk for baby, old man and patient.
Owner:YINGTAN HUABAO FLAVORS & FRAGRANCES
Acidophilus goat milk and method for making same
InactiveCN101081045AInhibition of reproductionRegulate stomachMilk preparationSoured milkMilk products
The present invention discloses one kind of sour ewe milk and its production process, and belongs to the field of sour milk producing technology. Fresh ewe milk or remade ewe milk as the material is produced into curd type sour ewe milk, stirred sour ewe milk or flavored sour ewe milk through purifying, standardizing, compounding, thickening, sterilizing, fermenting, cooling and other steps. These sour ewe milk products are healthful, and the production process is simple and suitable for industrial application.
Owner:张保钢
Color-protecting and fresh-keeping method for fresh-cut potatoes
InactiveCN103070226AAvoid quality impactExtended shelf lifeFruits/vegetable preservation by irradiation/electric treatmentFruits/vegetable preservation by freezing/coolingBiotechnologyChipped potatoes
The invention discloses a color-protecting and fresh-keeping method for fresh-cut potatoes. The method includes selecting fresh or stored potatoes as the raw material, washing cleanly, peeling, cutting into chips with thicknesses of 0.5-1.0mm, adding a composite color-protecting fresh-keeping solution having the same mass with the potato chips, and soaking for 10-30min at 20 DEG C-30 DEG C; then fishing out the potato chips from the composite color-protecting fresh-keeping solution, washing cleanly with water, soaking in water at 10-150w ultrasonic power and 10-50kHz ultrasonic frequency for 10-30min of ultrasonic time; fishing out the potato chips and draining off, processing for 10-30min under the condition of subzero 0.01MPa-subzero 0.09MPa of vacuum degree; and finally taking the potato chips out of the vacuum environment and soaking immediately into an arbutin solution with the concentration of 0.05-0.3mmol / L, soaking for 5-15min, taking the soaked potato chips out and draining for 1-3min, performing decompression packaging immediately, and storing at an environment of subzero 4 DEG C to zero DEGC. The method adopts ultrasonic and vacuum cooperative processing as well as the Chinese herb extract arbutin as the color-protecting fresh-keeping agent to inhibit enzyme and microbial activities, is better in effect compared with the prior art, and ensures that various potato processed products are prevented from brown stain in a cold storage process for more than 10 days.
Owner:SHANXI AGRI UNIV
Artemisinin derivatives freeze-dried preparation and preparation method
ActiveCN101125127AImprove securitySimple processPowder deliveryOrganic active ingredientsSolventCyclodextrin derivative
The present invention relates to a frozen dry preparation of drug artemisinin derivatives and the preparation method. The present invention is composed of artemisinin derivatives and water-soluble cyclodextrin derivatives, the weight ratio of the artemisinin derivatives and water-soluble cyclodextrin derivatives is 1: 30 to 70, the smashed artemisinin derivatives are added into the water solution of the water-soluble cyclodextrin derivatives at 50 DEG C to 90 DEG C for agitating and dissolving, then the frozen dry preparation is prepared by the conventional process of the injection and the frozen drying. The low-temperature micro-powder technology of the present invention has the advantages that the technology can ensure the physical and chemical properties of the artemisinin derivatives to be consistent before and after the smashing, the process is relatively simple compared with the prior art, the equipments make use of the existing production equipments of the company, thus saving the investment and shorten the production cycle; the dispensing solvent is the water for injection, without the organic solvents, surfactants and cosolvents, so as to improve the safety; the present invention is easy to be dissolved in water, and has fast dissolution speed and the infinite dilution stability, thus avoiding the secondary pollution caused in the other methods which firstly prepare the artemisinin derivatives into the inclusions by using the organic solvents and then sub-package the inclusions thereof.
Owner:KPC PHARM INC
External emplastrum for treating deficiency-cold for children
InactiveCN101036770ANo toxic performanceExcellent cure rateAerosol deliveryDigestive systemSkin complexionHiccups
The invention discloses an external plaster for pediatric deficiency cold and preparation method thereof. Aim at the physiological and pathological characteristics of children and the pathogenic factors and pathogenesis of pediatric deficiency cold, 8 Chinese crude drugs ccontaining white atractylodes rhizome, clove, chinese magnoliaving, nutmeg and so on, is processed by decocting, condensing, crushing, concocting and the other steps to form plaster. The inventive plaster has functions of invigorating qi and yang, supplementing huo and eliminating han. The principle indications are pediatric deficiency cold, sickness, hiccup, diarrhea, cllic, cough, night cry, enuresis, pale complexion, coldness of the body and limbs, tiredness, inappetence, pale lips, whitish tongue and thin white tongue, etc. The external plaster is safe and convenient in use, with remarkable effect. It solves the probleme of fearing the needle and repelling the drugs of the children, furthermore convenient for controling quality and favourable to large-scale industrialization and continuous production.
Owner:左耀武
External plaster for treating child cold diarrhea and preparing method
InactiveCN1899379ASignificant effectEasy to administerAerosol deliveryDigestive systemDiarrheaPediatrics
The present invention discloses a kind of externally applied plaster for treating children's cold type diarrhea and its preparation process. The externally applied plaster is developed based on children's physiological and pathological characteristics and the pathogenesis of cold type diarrhea, and is prepared with ten kinds of Chinese medicinal materials, including atractylodes rhizome, schizonepeta, bupleurum root, etc. and through decoction, concentration, crushing and other steps. It has the functions of invigorating spleen, eliminating wetness, regulating qi, etc. It has obvious curative effect on children's cold type diarrhea, use safety and convenient quality control.
Owner:赤峰市新州中药饮片有限责任公司
External emplastrum for curing food retention in child stomach and the preparing method
InactiveCN101036718APromote secretionPromote choleretic effectDigestive systemSheet deliveryEtiologyDisease
Disclosed are an external plaster for treating children retention syndrome and a preparing method thereof. The plaster is formed by 9 kinds of traditional Chinese medicines, e.g., hovenia dulcis, rhizoma atractylodis, radix bupleuri, etc., with steps of decocting, concentrating, crushing, concocting and the like based on physiological and pathological features of children and etiology and pathogenesis of the children retention syndrome. According to the invention, the prepared external plaster has curative effects of strengthening spleen and improving appetite, promoting digestion and relieving stasis, regulating qi and guiding stagnancy, and moving the bowels, which mainly treats diseases such as no appetite for milk, abdominal fullness and distention, bellyache, emesis, constipation, crying and irritability, reddened tongue, thick or greasy tongue, or fever, dyspneic cough, phlegm(or wheezzing sound), etc., caused by the children retention syndrome. The invention is convenient and safe for using, with remarkable effect and good compliance of children, in addition, the composition facilitates to control the quality thereof and is suitable to industrial scale continuous production.
Owner:INNER MONGOLIA DAMO PHARMA
Traditional Chinese medicine composition with effects of removing freckles and whitening and preparation and application methods thereof
InactiveCN107737272AEasy to useUse naturalCosmetic preparationsAnthropod material medical ingredientsSide effectBletilla striata
The invention discloses a traditional Chinese medicine formula with effects of removing freckles and whitening and a preparation method thereof and belongs to the technical field of human health careproducts. The traditional Chinese medicine formula contains the following raw materials: 4-18 parts of ganoderma lucidum, 9-36 parts of tricholoma matsutake, 3-12 parts of ginseng, 1-4 parts of bearberry leaf extract, 3-12 parts of rhizoma polygonati, 3-12 parts of bighead atractylodes rhizome, 1-6 parts of bletilla striata, 3-12 parts of white paeony root, 3-12 parts of ampelopsis japonica, 3-12parts of silkworm larva, 4-12 parts of poria cocos, 1-6 parts of angelica sinensis, 3-9 parts of paeonia lactiflora, 3-12 parts of astragalus, 1-5 parts of almond, 2-10 parts of semen coicis, 1-5 parts of fructus kochiae, 1-5 parts of cortex dictamni, 1-5 parts of liquorice and 2-6 parts of mint. The whitening and freckle removing skin care product provided by the invention is mild and non-irritating, can be applicable to population with sensitive skin, is simple and convenient to use, natural and zero in side effect and has excellent application prospects.
Owner:TONGJI UNIV
Makeup removing oil, and preparation method thereof
The invention discloses a makeup removing oil, and a preparation method thereof. The makeup removing oil is prepared from, by mass, 20 to 50 parts of tea seed oil, 20 to 35 parts of sweet apricot kernel oil, 10 to 25 parts of grape seed oil, 8 to 25 parts of isohexadecane, 10 to 22 parts of a pure plant emulsifying agent, 0 to 0.5 part of geranium essential oil, and 0.01 to 0.04 part of an antioxidant. Compared with the prior art, the makeup removing oil is an integral whole, is safe and mild via interaction of the ingredients and optimizing of a formula, is capable of removing makeup and dirt, is fresh and cool, is not greasy, is capable of killing bacteria and eliminating inflammation, and repairing skin; the preparation method is simple, and is suitable for industrialized production.
Owner:JIANGSU POLYTECHNIC COLLEGE OF AGRI & FORESTRY
Method for extracting dihydroquercetin form larch
InactiveCN101333204AFree radical scavengingInhibition of lesionsOrganic chemistryConiferophyta medical ingredientsLarchUnit/Kilogram
Disclosed is a preparation method for extracting dihydro quercetin from larch. The invention is an improved production method. Due to different production materials and extraction processes, the dihydro quercetin products in U.S. and European markets are very expensive, at a price of 200-1000 euros per gram. Such a high market price economically limits the wide range of practicability of dihydro quercetin products. During the pretreatment of raw materials, 50 kilograms of larch wood chips and 500-800 liters of 50% ethanol solution are added into an extractor to get fully stirred and mixed; then the mixture is extracted and filtered for the second extraction; the filtrates are combined and adsorbed; the organic solvents are collected and dried by a vacuum evaporator through organic phase vacuum distillation; according to a weight proportion of 10:1, the residues are dissolved with hot water, decolorized with activated carbon and then crystallized at a temperature of 3-5 DEG C to precipitate the dihydro quercetin product which is then crushed after vacuum drying to finished product. The invention is used to extract dihydro quercetin from larch.
Owner:黑龙江花旗科技发展有限公司 +2
External use plaster for treating child heat diarrhea and preparing method
InactiveCN1899455APromote secretionRegulates gastrointestinal motilityAnthropod material medical ingredientsAerosol deliveryVerbenaDiuresis
The present invention discloses a kind of externally applied plaster for treating infant heat diarrhea and its preparation process. The externally applied plaster is developed based on the physiological features of infant and pathological features and pathogenesis of infant heat diarrhea, and is prepared with 12 kinds of Chinese medicinal materials, including eucalyptus leaf, membrnaceous marshmarigold, European verbena, etc. and through decoction, concentrating, crushing, and other steps. The externally applied plaster has the functions of clearing away heat, promoting diuresis, regulating qi and middle Jiao, invigorating spleen and arresting diarrhea. It is used for treating infant heat diarrhea.
Owner:INNER MONGOLIA DAMO PHARMA
Levocarnitine for injection and preparation method thereof
ActiveCN102309475ANon-allergenicEfficient processOrganic active ingredientsMetabolism disorderActivated carbonPenicillin
The invention discloses a levocarnitine for injection and a preparation method thereof. The preparation method comprises the following steps: weighing sodium dihydrogen phosphate and sodium hydroxide, using injection water to dissolve the weighed sodium dihydrogen phosphate and sodium hydroxide and diluting and uniformly mixing, dissolving levocarnitine and mannitol in the buffer solution, addingneedle active carbon and stirring uniformly, stirring for adsorption, and using filtering membrane for filtering and removing carbon; carrying out fine filtration through 0.22 mum microporous membrane, after the intermediate passed the inspection, filling the filtrate with the amount of 5 ml in 15 ml penicillin bottle (using up the liquor in 6 h after degerming), and carrying out half-plug pressing, freeze-drying, capping, visual inspection, labeling, and packaging. According to the invention, buffer salts (the buffer salt ion pair consisting of sodium dihydrogen phosphate and sodium hydroxide) with buffer effect are used in stead of traditional hydrochloric acid to regulate the pH value of the levocarnitine solution, so that the main drug is always in a stable pH environment in the wholepreparation process, and the API degradation caused by violent change of pH value of levocarnitine is avoided. Compared with the similar kind products, the levocarnitine disclosed in the invention has higher effectiveness and higher safety.
Owner:长春海悦药业股份有限公司
Lactic acid bacteria goat milk beverage and preparing process thereof
InactiveCN101081046APrevents bloatingPrevent diarrheaMilk preparationLactic acid bacteriumSheep milk
The present invention discloses one kind of ewe milk beverage with lactic acid bacteria, and belongs to the field of beverage producing technology. Sour ewe milk as the main material is produced into the ewe milk beverage with lactic acid bacteria through mixing with water, sugar or sweetening agent, fruit juice, stabilizer and other components; filtering; sterilizing; homogenizing and other steps. The ewe milk beverage with lactic acid bacteria is healthful, and the production process is simple and suitable for industrial application.
Owner:DALIAN JIUYANG DAIRY
Hydrophilic polyurethane pressure-sensitive adhesive for skins, and preparation method thereof
ActiveCN104491922AGood initial adhesionStrong holding powerSurgical adhesivesAbsorbent padsFiberPoly(ethylene glycol) dimethyl ether
The invention provides a hydrophilic polyurethane pressure-sensitive adhesive for skins, and a preparation method thereof. The pressure-sensitive adhesive comprises polyurethane, sweetgum resin, alginate, gelatin, fibrous proteins, plant polysaccharides, methacryloylethyl sulfobetaine, triethyl citrate and polyethylene glycol dimethyl ether. The preparation method comprises the following steps: weighing all the above components according to weight parts; mixing polyurethane with sweetgum resin, alginate and gelatin in an open mill, adding the fiber proteins, the plant polysaccharides, methacryloylethyl sulfobetaine, triethyl citrate and polyethylene glycol dimethyl ether, continuously mixing for uniform dispersion, and carrying out hot pressing by using a plate vulcanizing machine to form the pressure-sensitive adhesive with desired thickness. Compared with pressure-sensitive adhesives in the prior art, the acrylic medical pressure-sensitive adhesive prepared in the invention has the advantages of good initial tack, low peel strength, no sensitization, no residue and very strong permanent adhesion.
Owner:WEIXIN INDAL & TRADE GUANGZHOU
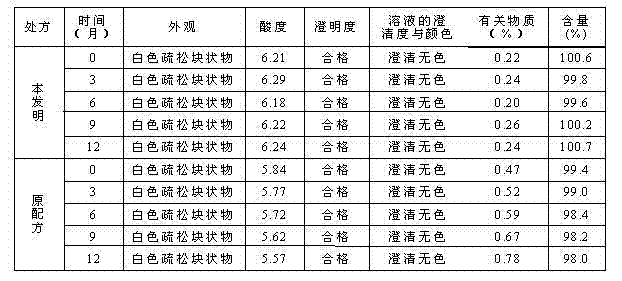
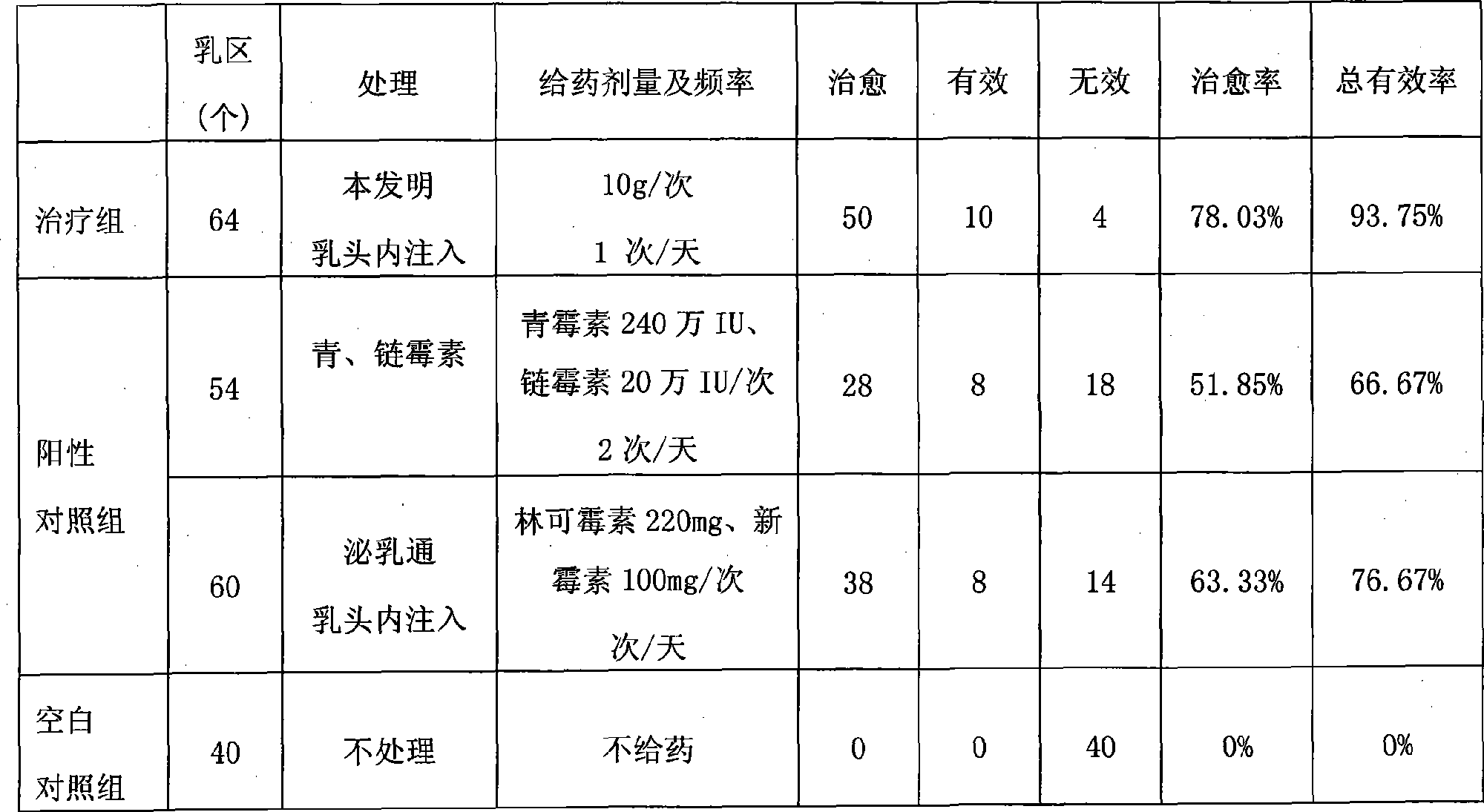
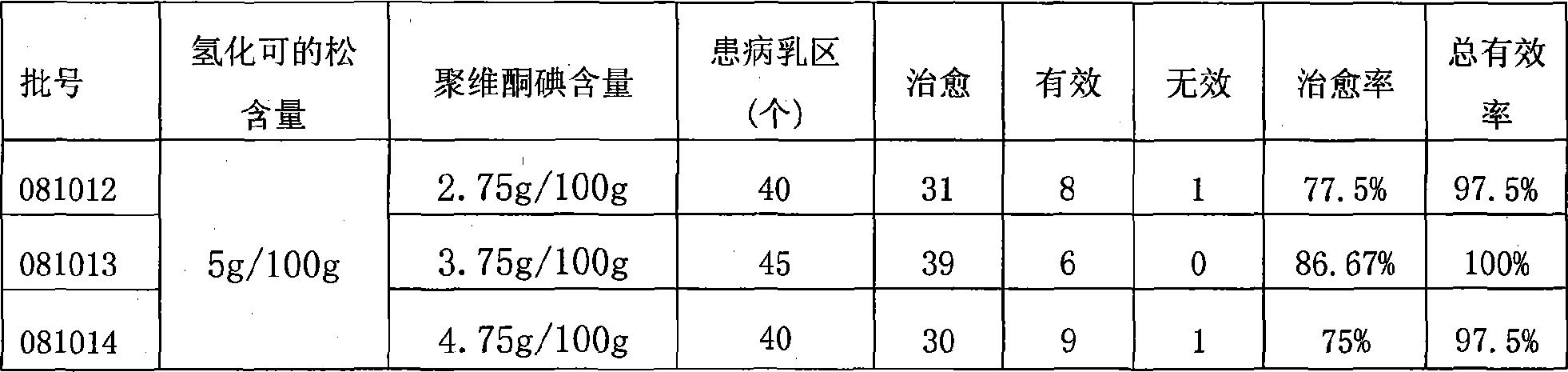
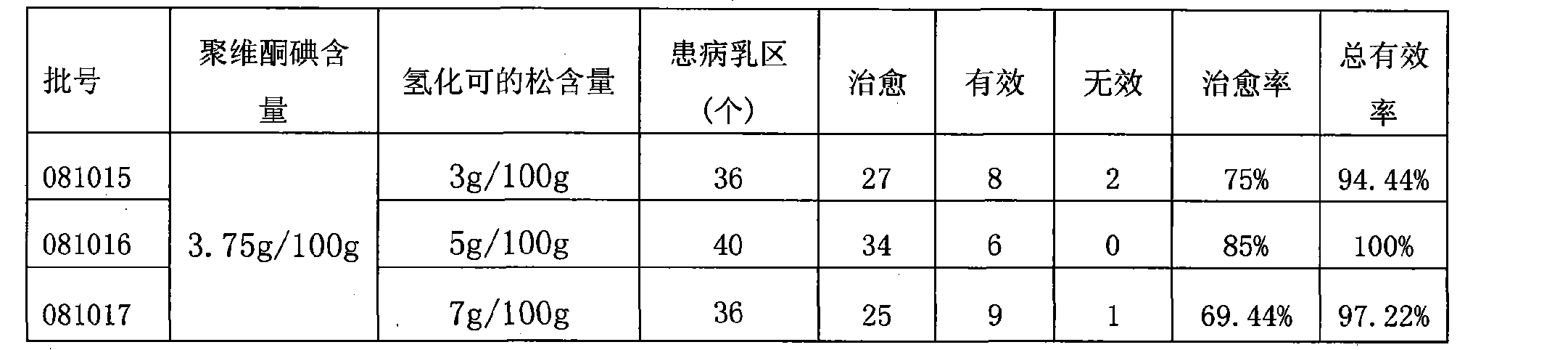
Compound povidone iodine gel for treating mastitis of milk cattle as well as preparation method and application
InactiveCN101439044AStrong bactericidal powerNon-allergenicAntibacterial agentsOrganic active ingredientsSensitizationGlycerol
The invention belongs to the technical field of animal remedy preparation and in particular relates to a pharmaceutical composition for curing dairy cow mastitis and a preparation method and a usage of the pharmaceutical composition. The remedy comes into being by mixing carbomer-940, povidone iodine, hydrocortisone, potash iodate, propylene glycol or glycerol, sodium hydroxide and purified water. The povidone iodine in the preparation has great sterilizing power, no odor or stimulation or sensitization and low toxicity and leads to no residuals which are formed in milk and harmful to human body, no withdrawal period is required during the course of medication, and no milk drinking stop is required; a prepared gel preparation is injected into breasts, a relatively high drug concentration can be achieved in the breasts in a short time, and the treatment cycle is short. Results of clinical trials show that the cure rate of recessive mastitis of the diary cow is up to 86.43 percent, and the effective rate is up to100 percent; and the cure rate of clinical mastitis of the diary cow is up to 78.03 percent, and the effective rate is up to 93.75 percent.
Owner:HUAZHONG AGRICULTURAL UNIVERSITY
Medicinal composition for treating gynaecologic phlogosis, its production and quality controlling method
ActiveCN101028384ANo obvious side effectsObvious side effectsAnthropod material medical ingredientsAntipyreticIrritationSkin irritant
A Chinese medicine for treating trichomonas vaginitis, non-specific vaginitis, pudendal itching, etc is prepared from 7 Chinese-medicinal materials including earthworm, flavescent sophora root, cnidum fruit, cloves, etc. Its preparing process and its quality control method are also disclosed.
Owner:江西杏林白马药业股份有限公司
Method for preparation of egg white oligopeptide having high F value
ActiveCN101012472AHas the effect of protecting the liverReduce odorFermentationPre treatmentOligopeptide
The invention discloses a making method of high-F value egg oligopeptide, which comprises the following steps: a. predisposing raw material; b. hydrolyzing protein for two steps; c. adsorbing through active carbon; d. desalting; obtaining the product with molecular weight at 300-1000 and F value more than 20.
Owner:JILIN JINYI FOOD CO LTD
Medical cold-compressing plaster and preparation method thereof
InactiveCN109394737AEasy to useDoes not affect movementPeptide/protein ingredientsAntipyreticSide effectBiocompatibility Testing
The invention provides a medical cold-compressing paster which comprises a back lining layer, a gel layer and a covering layer. The gel layer is prepared from raw materials, the raw materials include,by mass, 2%-15% of a polymer substance, 2%-10% of an epidermal growth factor, 2%-8% of ceramide, 3%-8% of a moisturizer, 3%-10% of a radix bupleuri extract, 2%-8% of a dandelion extract, 3%-12% of agolden cypress extract, 5%-10% of an aloe extract, 2%-10% of a honeysuckle flower extract and the balance purified water. The invention also provides a preparation method of the medical cold-compressing paster. The medical cold-compressing paster has the advantages that the medical cold-compressing paster is convenient to use; the medical cold-compressing paster achieves both cold-compressing cooling and pain relieving, also promotes organization healing; the biocompatibility is good, and the medical cold-compressing paster does not have sensitization and irritation; the drug release performance is good, and the application time is long; the medical cold-compressing paster provides different shapes, and the different demands are met; the preparation method is easy to operate, the raw materials are wide in source, the preparation cost is low, no toxic-side effect is produced, and the medical cold-compressing paster is safe and reliable.
Owner:SHANDONG ZHUSHI PHARMA GRP CO LTD
Skin-care sunblock product for infants
InactiveCN105055249AHigh affinityImprove microcirculationCosmetic preparationsToilet preparations1,3-PropanediolDimethyl siloxane
The invention provides a skin-care sunblock product for infants. The skin-care sunblock product comprises an A phase, a B phase, a C phase and a D phase. The A phase is composed of cetostearyl alcohol olive oil acid ester, cetostearyl alcohol, glyceryl stearate, squalane, simmondsia chinensis seed oil, isohexadecane, nano-zinc oxide, ethylhexyl methoxycinnamate and polydimethylsiloxane. The B phase is composed of water, glycerol, 1,3-propylene glycol, butanediol and EDTA disodium. The C phase is emulgator polyacrylate-13, polyisobutene and polysorbate-20. The D phase is composed of vitamin E, phenoxyethanol, 1,2-octylene glycol and plant desensitizer. The skin-care sunblock product for infants is capable of protecting the delicate skin of the infants and protecting the infants against sunburn, good in moisturizing effect, safer and healthier.
Owner:YANGZHOU ZHONGHUI BIOTECH
Freckle-removing essence
InactiveCN104352395AHas super solubility propertiesHigh activityCosmetic preparationsToilet preparationsSophocarpidineIrritability
The invention relates to a freckle-removing essence, which is not only capable of slowly fading freckles on faces of people, but also capable of enabling skin on the faces to become beautiful and white slowly. On the basis of A, B, D and E basic formulae, a C raw material is added; and furthermore, the C raw material is composed of ginsenoside extracting solution, pseudo-ginseng extracting solution, licoflavone extracting solution, Chinese angelica extracting solution and lucid ganoderma extracting solution. Compared with the prior art, the freckle-removing essence disclosed by the invention has the advantages as follows: because a strong penetrating agent, namely azone, and an antihistamine reagent, namely sophocarpidine, are used in the formula, the freckle-removing essence prepared by the invention is rapid to take effect and free from irritability; raw materials are convenient to buy and low in price; the production process is simple, therefore, the production cost of the freckle-removing essence is low; no any hormone or other substances hazardous to skin exist in the prescription, so that skin cannot be injured in the use process; and thus, the freckle-removing essence is welcome by the masses of users.
Owner:AESTHETIC TECH BEIJING +2
Propranolol hydrochloride submicron emulsion gel and preparation method and application thereof
ActiveCN105434337ANo absorptionPromote absorptionOrganic active ingredientsAerosol deliverySide effectEmulsion
The invention discloses propranolol hydrochloride submicron emulsion gel and a preparation method and application thereof. The propranolol hydrochloride submicron emulsion gel comprises propranolol hydrochloride and pharmaceutical acceptable medicinal excipients, wherein the medicinal excipients comprise oil, a surfactant, a cosurfactant, a penetration enhancer, a gel substrate, a pH conditioning agent and the balance water. The propranolol hydrochloride submicron emulsion gel can efficiently deliver the propranolol hydrochloride to skin tissue to enable drugs to be released slowly, skin targeted drug delivery treatment on superficial infantile hemangioma is achieved, and the problems that for an existing drug, the curative effect is low, the side effect is large, and the transdermal absorption ratio is low are effectively solved.
Owner:WUHAN CONFORM PHARMA CO LTD +1
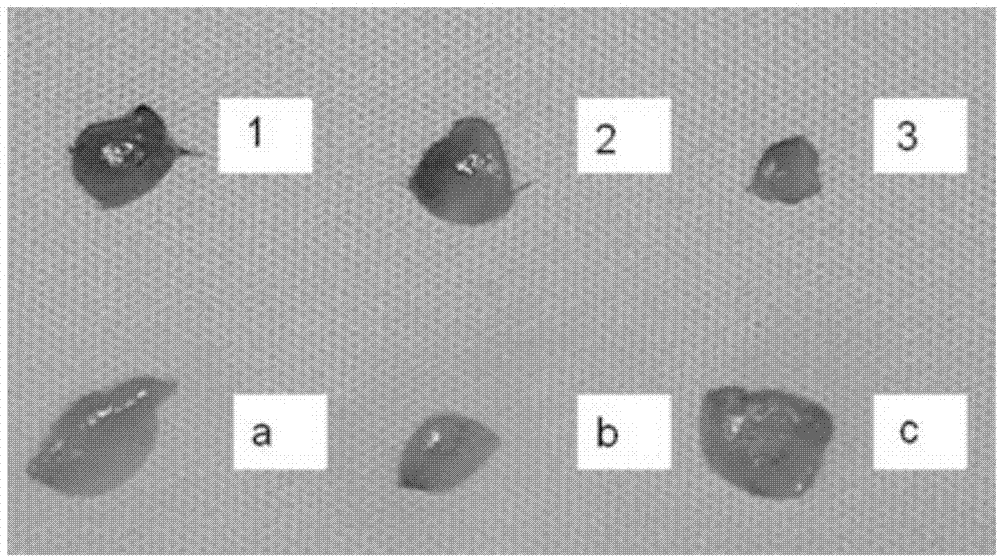
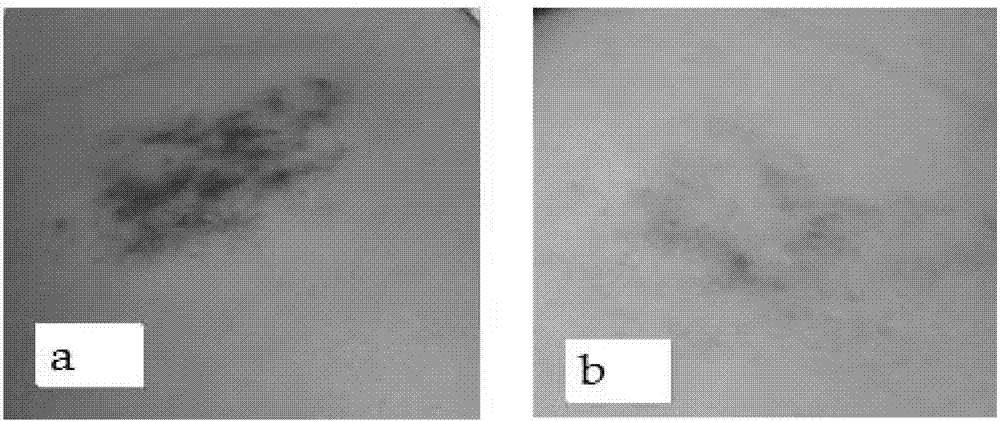
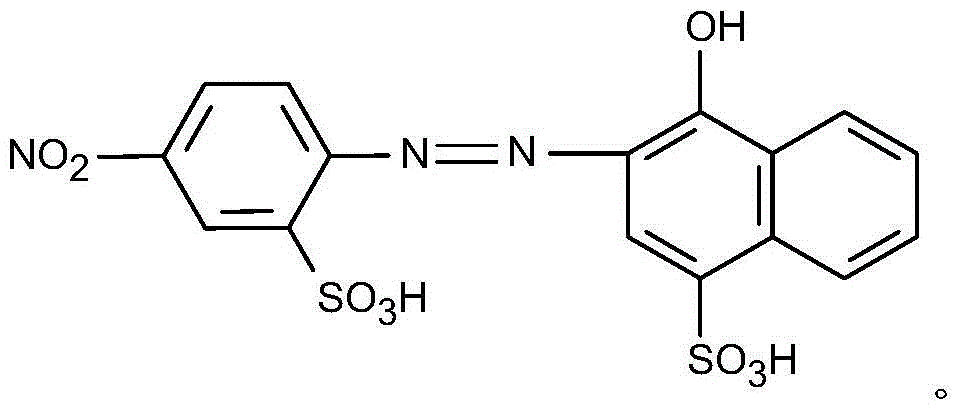
Novel environment-friendly acid blue-light red dye applied to furs and synthesis method of dye
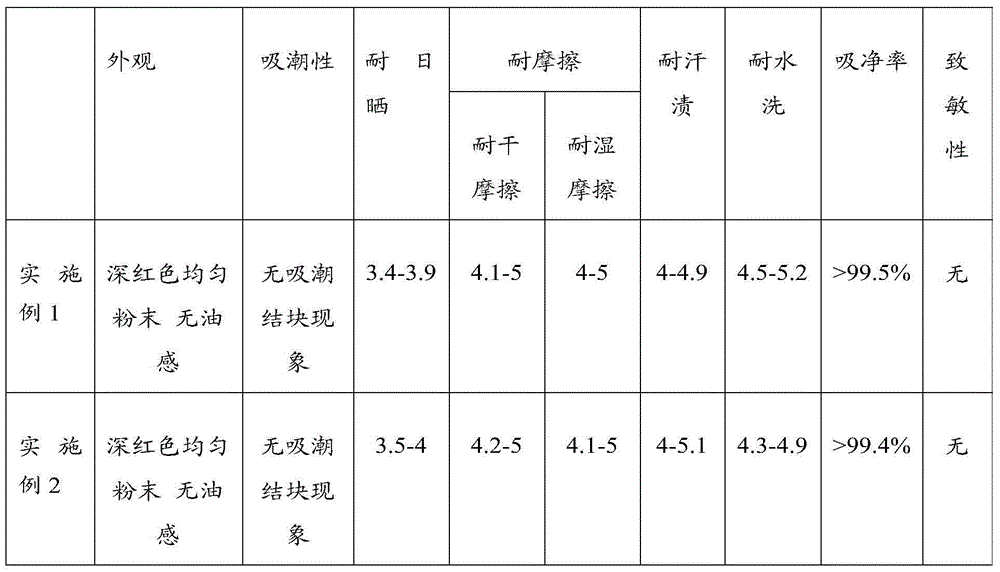
ActiveCN104448895ANon-allergenicFull colorMonoazo dyesDyeing processEnvironmental resistanceSynthesis methods
The invention relates to the field of processing processes of animal furs and in particular relates to a novel environment-friendly acid blue-light red dye applied to furs and a synthesis method of the dye. The structural formula of the novel environment-friendly acid blue-light red dye is as shown in the description. The method comprises the following steps: (1) carrying out diazo-reaction; and (2) coupling. The dye can be widely used for dyeing various furs. The blue-light red dye has a series of advantages of high dyeing degree, high net adsorption rate, good compatibility and full color and luster; meanwhile, the dye is free of 24 aromatic hydrocarbons banned by European Union; the dye is environmentally friendly; by tests, the dye is free of sensitization; the safety is ensured.
Owner:BEIJING FANBO CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com