Gene recombination human collagen fusion peptide segment, preparation method and application thereof
A human collagen and gene recombination technology, applied in the biological field, can solve the problems of biosafety and biocompatibility, and the human body is not identical.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
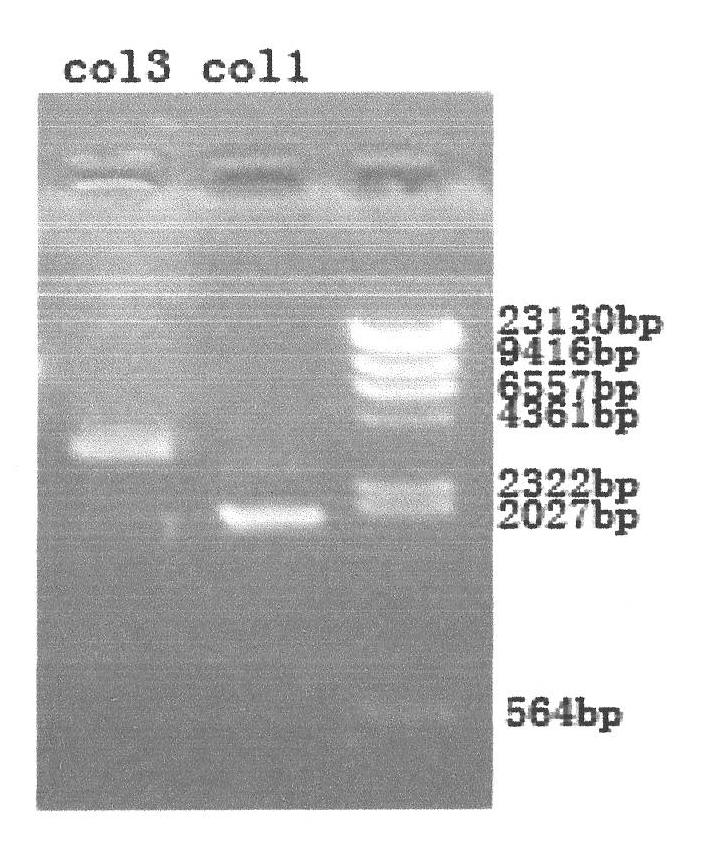
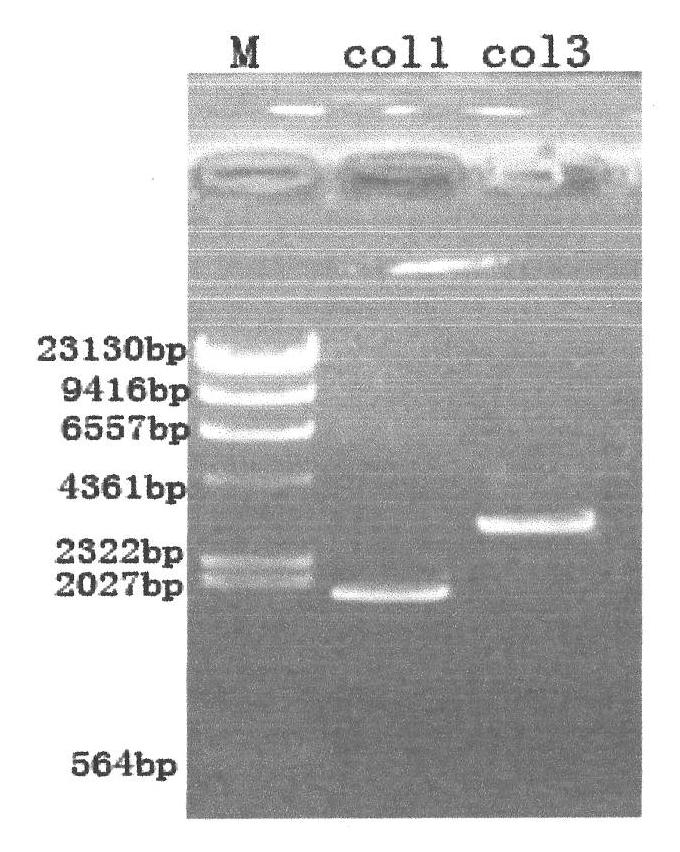
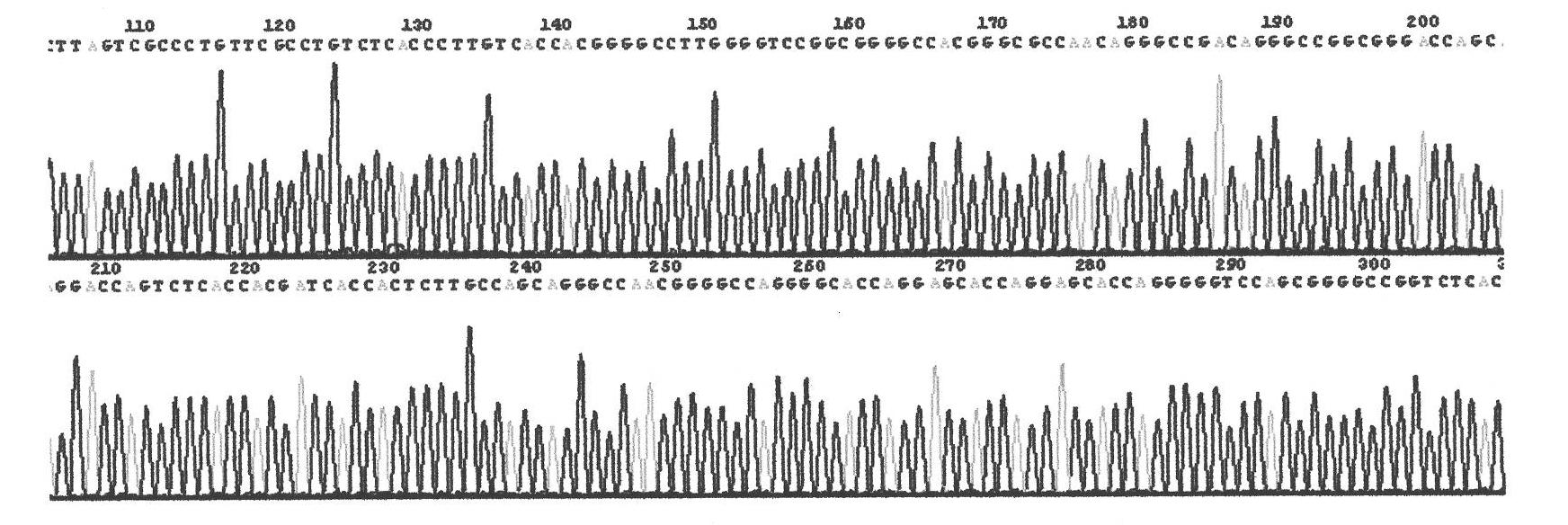
[0139] The total length of the gene recombinant human collagen fusion peptide is 839 amino acids, and its nitrogen terminal is the 908-1137 peptide of type III human collagen, a total of 229 amino acids, and its carbon terminal is the 497-1104 peptide of type I human collagen A total of 608 amino acids, the two peptides are linked by glutamic acid and phenylalanine; the amino acid sequence of the recombinant human collagen fusion peptide is as follows:
[0140] AGNTGAPGSP GVSGPKGDAG QPGEKGSPGA QGPPGAPGPL GIAGITGARG
[0141] LAGPPGMPGP RGSPGPQGVK GESGKPGANG LSGERGPPGP QGLPGLAGTA 100
[0142] GEPGRDGNPG SDGLPGRDGS PGGKGDRGEN GSPGAPGAPG HPGPPPGVGP
[0143] AGKSGDRGES GPAGPAGAPG PAGSRGAPGP QGPRGDKGET GERGAAGIKG 200
[0144] HRGFPGNPGA PGSPGPAGQQ GAIGSPGPAE FGADGVAGPK GPAGERGSPG
[0145] PAGPKGSPGE AGRPGEAGLP GAKGLTGSPG SPPGDGKTGP PGPAGQDGRP 300
[0146] GPPGPPGARG QAGVMGFPGP KGAAGEPGKA GERGVPGPPG AVGPAGKDGE 350
[0147] AGAQGPPGPA GPAGERGEQG PAGSPGFQGL PGPAGPPGEA GKPGEQGVPG 4...
Embodiment 2
[0377] The recombinant human collagen fusion peptide prepared in Example 1 of the present invention is used to prepare cosmetics, which are made of the following raw materials in mass ratio:
[0378] Recombinant Human Collagen Fusion Peptide 10g
[0379] Hyaluronic Acid 0.5g
[0380] Glycerin 25g
[0381] Deionized water 1000g.
[0382] Its preparation method is as follows:
[0383] Take dry powdered gene recombinant human collagen fusion peptide, hyaluronic acid, glycerin, dissolve in deionized water, adjust the pH value to 6.5-7.5, and filter it with a 0.22um filter membrane in a sterile environment to obtain colorless and transparent Liquid, sealed in 5ml glass bottle or plastic bottle by aseptic method, stored in refrigerator at 4°C.
Embodiment 3
[0385] The recombinant human collagen fusion peptide prepared in Example 1 of the present invention is used to prepare cosmetics, which are made of the following raw materials in mass ratio:
[0386] Recombinant Human Collagen Fusion Peptide 8g
[0387] Hyaluronic Acid 0.3g
[0388] Glycerin 20g
[0389] Deionized water 1000g.
[0390] Its preparation method is identical with embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com