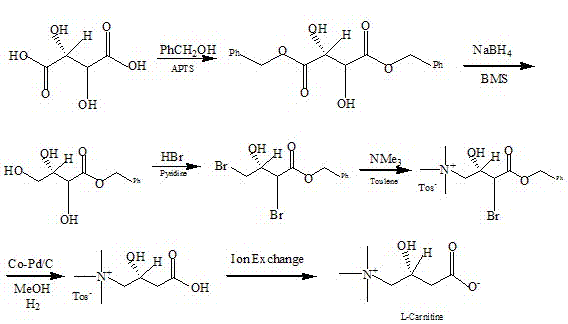
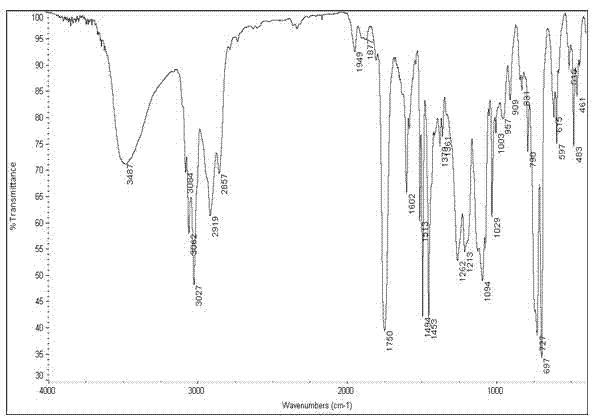
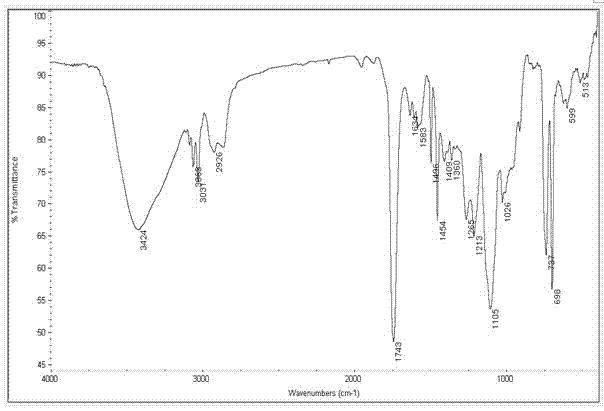
Method for synthesizing levocarnitine by taking D-(-)-tartaric acid as raw material
A technology of tartaric acid and dibenzyl tartrate, applied in chemical instruments and methods, preparation of organic compounds, chemical recovery, etc., to achieve the effects of low cost, mild reaction conditions, and simple process technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0050] Synthesis of D-(-)-dibenzyl tartrate:
[0051] Add 45g of D-(-)-tartaric acid, 2.25g of boric acid, 90ml of benzyl alcohol and 120ml of toluene solution into a 500ml three-necked flask. Toluene is the water-carrying agent. Stir and heat up until all the solids are dissolved, then heat up to reflux, and reflux at 116°C Divide the water, react until the water volume in the water separator no longer increases, record the water division. After cooling to room temperature, pour the reaction solution into a separatory funnel, add an equal volume of saturated NaHCO3 solution to wash until no bubbles are formed, and then wash the oil layer with distilled water for 2 to 3 times, let it stand for layers, and use anhydrous magnesium sulfate to treat the ester layer. Carry out drying, leave standstill overnight, filter, and filtrate is poured into distillation bottle and carries out normal pressure distillation, removes toluene, then decompression distillation removes benzyl alcoho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com