A pharmaceutical composition treating severe high-altitude diseases
A composition and high altitude sickness technology, which can be used in drug combinations, blood diseases, pharmaceutical formulations, etc., and can solve the problems of lack of severe altitude sickness and no reports of clinical application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment one: Observation of the effect of intravenous injection of levocarnitine and trimetazidine hydrochloride in different dose ratios on normal pressure hypoxia in mice
[0019] Trimetazidine hydrochloride: 0.75, 2.25, 4.5 mg / kg, approximately equivalent to human daily dose of 5, 15, 30 mg;
[0020] Levocarnitine: 450mg / kg, approximately equivalent to a daily human dose of 3g
[0021] Select 40 male mice, body weight 20±2g, randomly divide them into 4 groups according to body weight, 10 mice in each group, administer by tail vein injection of 10ml / kg, and give equal volume of normal saline to the blank control group, 1 time / day, for 7 consecutive days. 1 h after the last administration, put the mice in each group into a 160ml jar with 5g of soda lime added in advance, one for each bottle, and then smear the bottle cap with vaseline and seal it tightly. mouse survival time [Zheng Yue, Ji Yang. Animal models and anti-hypoxic drugs commonly used in anti-hypoxia ...
Embodiment 2
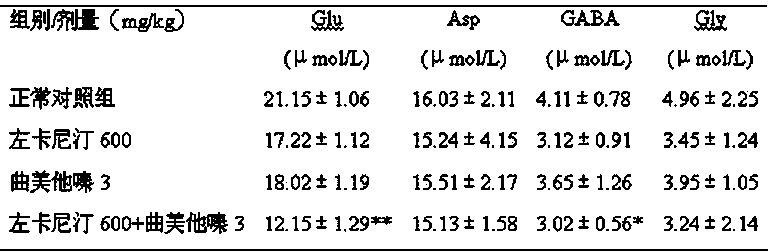
[0026] Embodiment two: Effects of levocarnitine and trimetazidine hydrochloride (200:1), injection administration, single and compound on acute cerebral hypoxia model in mice
[0027] 40 male Kunming mice were randomly divided into 4 groups: normal control group, levocarnitine 600mg / kg group, trimetazidine hydrochloride 3 mg / kg group, levocarnitine 600+trimetazidine hydrochloride 3mg / kg kg group, 10 rats in each group. 10ml / kg injection, the normal control group was given an equal volume of normal saline, once a day, for 7 consecutive days. One hour after the last administration, the mice were quickly decapitated from behind the ears, and the time from the decapitation to the cessation of gasping for breath was recorded immediately according to a stopwatch. The results are shown in Table 2.
[0028] Table 2 Effects of single and compound prescriptions on the acute cerebral hypoxia model in mice (n=10, ± S )
[0029]
[0030] Note: Compared with normal control group, ...
Embodiment 3
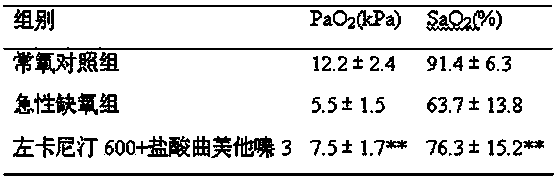
[0038] Embodiment three: Observation of the effect of levocarnitine and trimetazidine hydrochloride (200:1) on hypobaric hypoxia in rats
[0039]Set the dose of levocarnitine+trimetazidine hydrochloride to 600+3mg / kg.
[0040] Select 30 Wister rats, weighing 150g to 190g, and randomly divide them into 3 groups: normoxia control group: raise and collect materials in plain areas; acute hypoxia group: place animals in a hypobaric oxygen chamber, and the oxygen partial pressure in the chamber is 11.01 Kpa (approximately equivalent to the partial pressure of oxygen at an altitude of 5000m) after continuous decompression and hypoxia for 3 days, and then placed in a hypobaric oxygen chamber with an oxygen partial pressure of 13.25Kpa (approximately equivalent to the partial pressure of oxygen at an altitude of 4000m) to collect specimens [Zheng Yue, Ji Yang. Animal models and anti-hypoxic drugs commonly used in anti-hypoxia research. PLA Pharmaceutical Journal, 2010, 26 (2): 170~1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com