Cyclosporine A micelle eye drop and preparing method thereof
A technology of cyclosporine and eye drops, applied in the direction of cyclic peptide components, capsule delivery, pharmaceutical formulations, etc., can solve the problems of unsatisfactory safety and economical efficiency, poor intraocular permeability, poor patient compliance, etc., to achieve The effect of prolonging the drug action time, reducing the number of administrations, and reducing the concentration of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
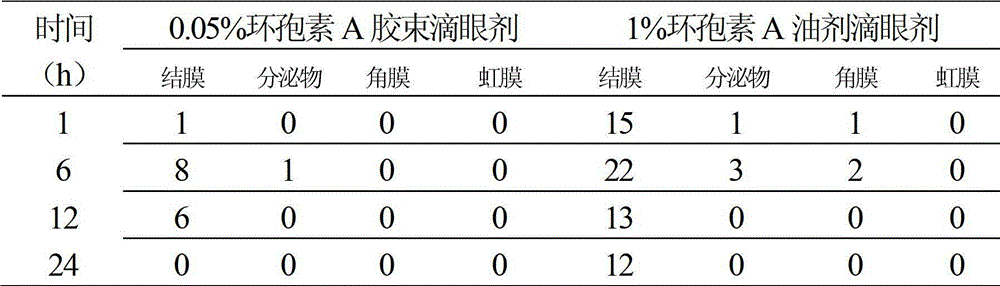
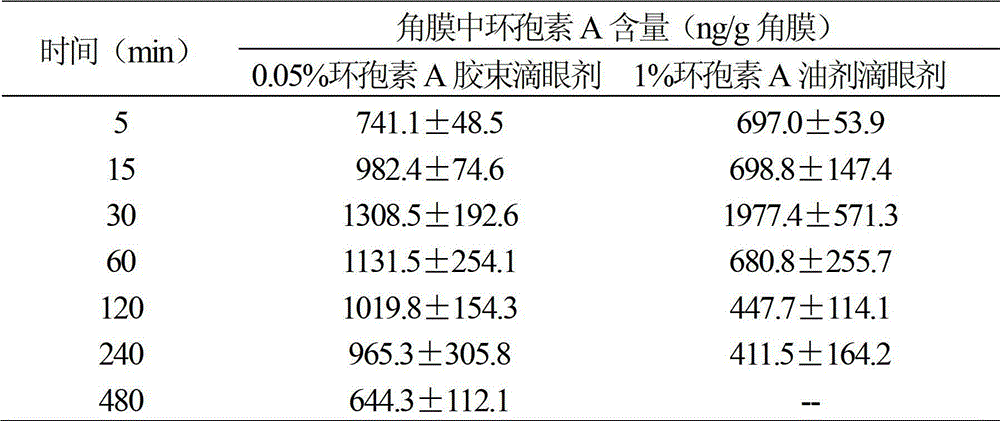
[0013] Put 5 mg of cyclosporine A and 150 mg of polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol (molecular weight: 50,000) in a 100 mL round-bottomed flask, add 10 mL of absolute ethanol, fully dissolve, and evaporate in a water bath at 40°C in a rotary vacuum. Water and ethanol to obtain a uniformly dispersed film of drug and copolymer, then add distilled water to fully wash the film, 300w power water bath ultrasonic 10min, to obtain a micellar solution. The micellar solution was passed through a 0.22 μm microporous membrane, and then freeze-dried to obtain the micellar powder. Then disperse and dissolve the micellar powder with 10 mL of borate buffer solution, add preservative benzalkonium chloride, and isotonic regulator sodium chloride, adjust the pH to 7.3, and then sterile filter and repack. The average particle size of cyclosporine A micelles measured by the laser particle size analyzer is 73nm, the dispersion index PDI=0.067, and the dispersion uniformity i...
Embodiment 2
[0015] Put 10 mg of cyclosporine A and 200 mg of polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol (molecular weight: 100,000) in a 100 mL round bottom flask, add 10 mL of dichloromethane, fully dissolve, and evaporate in a water bath at 40°C in a rotary vacuum for two Chloromethane to obtain a uniformly dispersed film of drug and copolymer, then add distilled water to fully wash the film, 300w power water bath ultrasonic 10min, to obtain a micellar solution. The micellar solution was passed through a 0.22 μm microporous membrane, and then freeze-dried to obtain the micellar powder. Then use 20mL phosphate buffer to disperse and dissolve the micellar powder, add preservative benzalkonium chloride, and isotonic regulator sodium chloride, adjust the pH to 7.3, and then sterile filter and repack. The average particle size of cyclosporin A micelles measured by laser particle size analyzer is 86nm, the dispersion index PDI=0.200, and the dispersion uniformity is good. Th...
Embodiment 3
[0017] Put 25mg of cyclosporine A and 300mg of polyethylene caprolactam-polyvinyl acetate-polyethylene glycol (molecular weight: 150,000) in a 500mL round bottom flask, add 30mL of dichloromethane, fully dissolve, and evaporate in a water bath at 40°C for two Chloromethane to obtain a uniformly dispersed film of drug and copolymer, then add distilled water to fully wash the film, 300w power water bath ultrasonic 10min, to obtain a micellar solution. The micellar solution was passed through a 0.22 μm microporous membrane, and then freeze-dried to obtain the micellar powder. Disperse and dissolve the micellar powder with 50 mL of phosphate buffer, add preservative benzalkonium chloride, and isotonicity regulator sodium chloride, adjust the pH to 7.3, and then sterile filter and repack. The average particle size of cyclosporine A micelles measured by the laser particle size analyzer is 110nm, the dispersion index PDI=0.162, and the dispersion uniformity is good. The cyclosporine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com