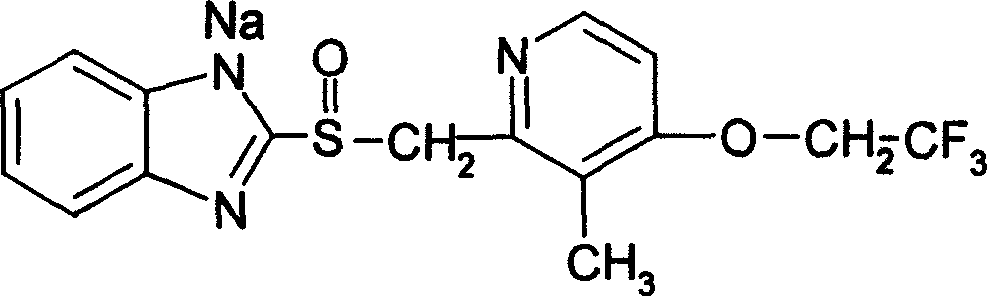
Enteric coated preparation of lansoprazole sodium and preparation method thereof
A technology for lansoprazole sodium and enteric-coated preparations is applied in the field of lansoprazole sodium enteric-coated preparations and the preparation thereof, and can solve the adverse effects of light, humidity and heat on drugs, inconvenient storage and transportation, and reduced drug stability. problems, to achieve the effect of easy control of product quality, easy storage and transportation, and improved drug stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Take 15~30g of lansoprazole sodium, 5~25g of disintegrant and 150~300g of diluent, pulverize and sieve, mix well, add 20~110g of wetting agent or binder as soft material, sieve for granulation, Vacuum dry at 30~50℃, sizing, add 5~15g of lubricant and mix, φ=0.5~1.0mm concave punching tablet, take 30~100g of coating agent, spray the isolation layer and enteric coating respectively to obtain Lanso Prazole sodium enteric-coated tablets. The diluent refers to any one or any two or any three of mannitol, crospovidone, lactose, starch, microcrystalline cellulose, and sucrose. The binder or wetting agent refers to any one or any of acrylic resin No. I to IV, poloxamer, hydroxypropyl methyl cellulose, microcrystalline cellulose, polyvinyl pyrrolidone Two or any three. The disintegrant refers to one to three types of sodium lauryl sulfate, methyl cellulose, povidone, modified starch, and hydroxyethyl methyl cellulose. The lubricant refers to any one or any two or any three of steary...
Embodiment 2
[0020] 1. the core
[0021] Lansoprazole sodium (main medicine) 18.4~36.8g
[0022] Starch (disintegrant) 25~50g
[0023] Mannitol (diluent) 15~30g
[0024] Lactose (diluent) 15~30g
[0025] Hypromellose 8% solution (adhesive, wetting agent) 15~30g
[0026] Magnesium stearate (lubricant) 3~10g
[0027] 1000 pieces
[0028] 2. Isolation layer
[0029] Hypromellose (coating agent) 082~1.63g
[0030] Titanium dioxide (coating agent) 0.15~0.30g
[0031] Tween-80 (coating agent) 5~15g
[0032] 1000 pieces
[0033] 3. Enteric coating layer
[0034] Polyacrylic resin II (enteric coating agent) 2.9~5.90g
[0035] Polyacrylic resin III (enteric coating agent) 0.72~1.44g
[0036] 95% ethanol 5~30g
[0037] Tween-80 (solubilizer) 3~10g
[0038] 1000 pieces
[0039] Weigh lansoprazole sodium and starch, mannitol, lactose according to the prescription, crush and sieve, mix well, add 8% hypromellose solution as soft material; 16# sieve for granulation; 30~50℃ vacuum Dry, granulate; mix with mag...
Embodiment 3
[0041] 1. the core
[0042] Lansoprazole sodium (main medicine) 18.4~36.8g
[0043] Starch (disintegrant) 20~40g
[0044] Mannitol (diluent) 25~50g
[0045] Lactose (diluent) 15~30g
[0046] Hypromellose 3% solution (adhesive, wetting agent) 10~40g
[0047] Magnesium stearate (lubricant) 5~15g
[0048] 1000 pieces
[0049] 2. Isolation layer
[0050] Hypromellose (coating agent) 0.81~1.63g
[0051] Titanium dioxide (coating agent) 1.5~3.0g
[0052] Tween-80 (coating agent) 3~10g
[0053] 1000 pieces
[0054] 3. Enteric coating layer
[0055] Polyacrylic resin II (enteric coating agent) 5.45~10.90g
[0056] Polyacrylic resin III (enteric coating agent) 1.22~2.44g
[0057] 95% ethanol 10~50g
[0058] Tween-80 (solubilizer) 5~15g
[0059] 1000 pieces
[0060] Weigh lansoprazole sodium and starch, mannitol, lactose according to the prescription, crush and sieve, mix well, add 3% hypromellose solution as soft material; 16# sieve for granulation; 30~50℃ vacuum Dry, granulate; mix with mag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com