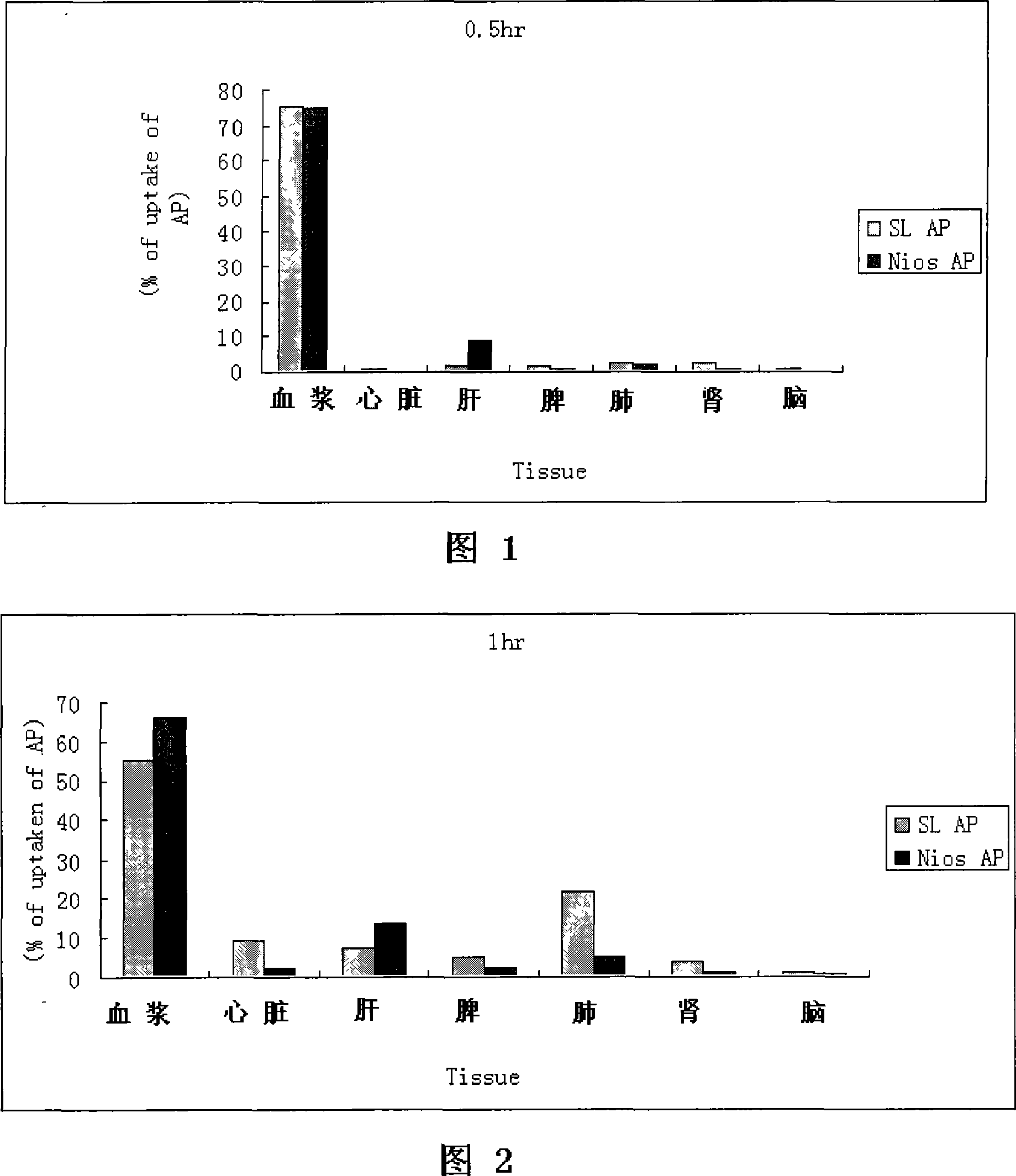
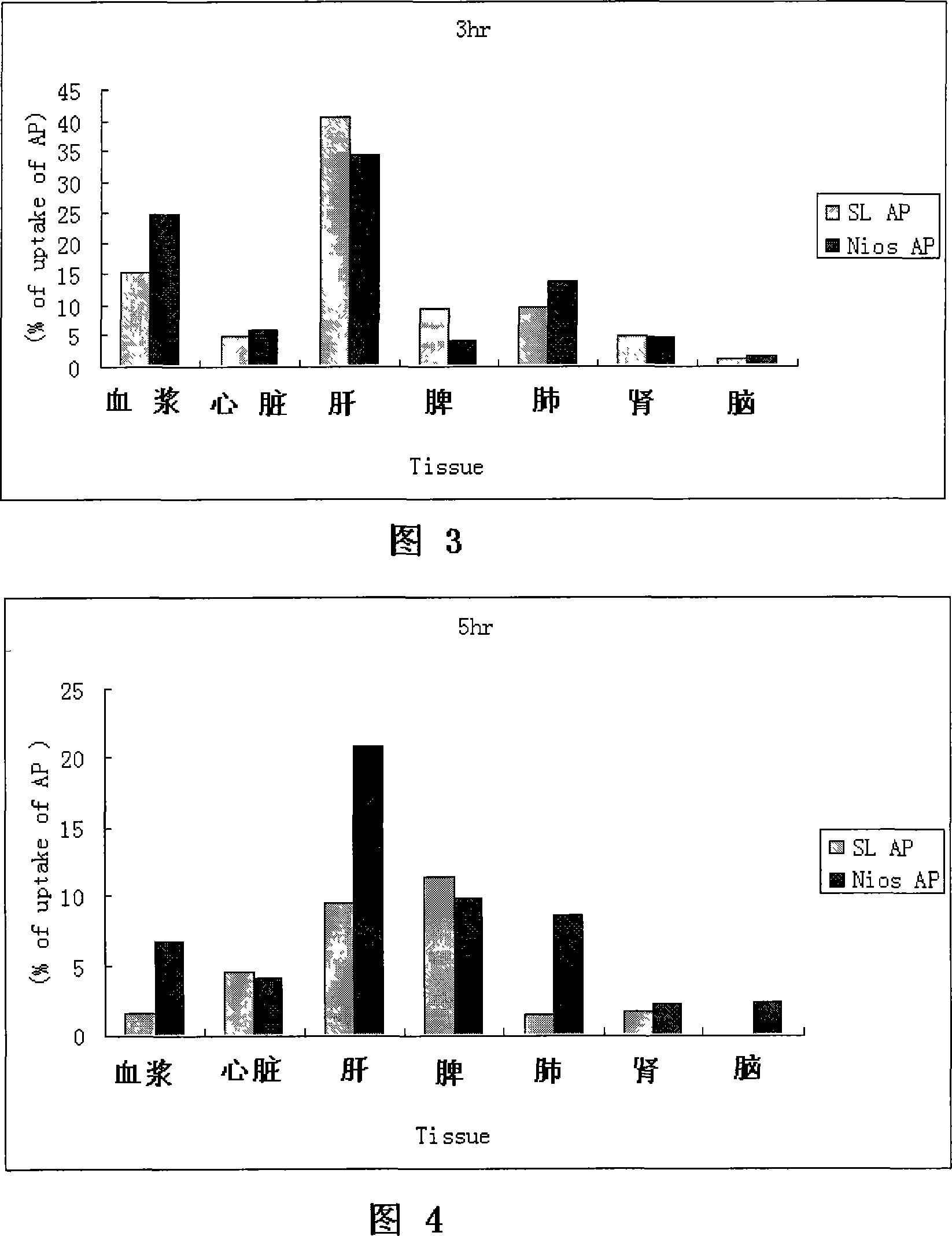
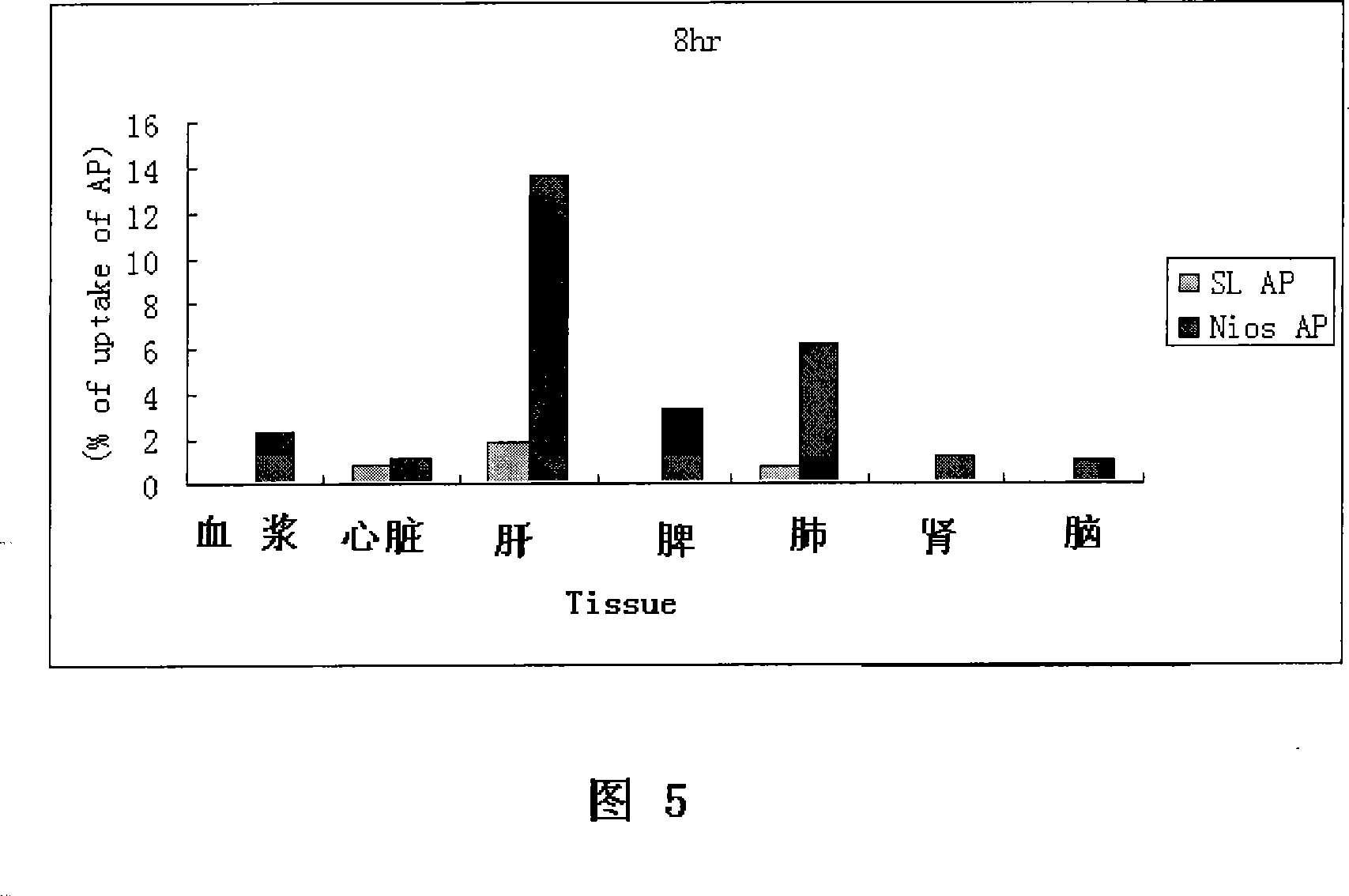
Apigenin vesicle preparation and its preparation technology
A technology of apigenin and vesicles, which is applied in the field of medicine, can solve the problems that have not been reported in domestic and foreign literature, and achieve the effects of improving bioavailability, obvious slow-release effect, and high encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the preparation of apigenin vesicle
[0030] prescription:
[0031] Apigenin 0.05g
[0032] Tween 80 0.5g
[0033] Cholesterol 0.25g
[0034] Proper amount of ethanol
[0035] Phosphate buffer at pH 7.4
[0036] Makes 100mL
[0037] Preparation process: Weigh the prescribed amount of apigenin, Tween 80 and cholesterol into a beaker, add an appropriate amount of ethanol to dissolve, and inject it into a phosphate buffer solution with a pH of 7.4 in a water bath at 55°C to prepare an apigenin vesicle solution. The encapsulation efficiency of the obtained vesicles was determined, and the encapsulation efficiency was 65.80%.
Embodiment 2
[0038] Embodiment 2: the preparation of apigenin vesicle
[0039] prescription:
[0040] Apigenin 0.05g
[0041] Span80 0.4g
[0042] Cholesterol 0.20g
[0043] Proper amount of ethanol
[0044] Phosphate buffer at pH 7.4
[0045] Makes 100mL
[0046] Preparation process: same as above, encapsulation efficiency is 60.68%.
Embodiment 3
[0047] Embodiment 3: the preparation of apigenin vesicle
[0048] prescription:
[0049] Apigenin 0.05g
[0050] Maize 0.50g
[0051] Cholesterol 0.20g
[0052] Proper amount of ethanol
[0053] Phosphate buffer at pH 7.4
[0054] Makes 100mL
[0055] Preparation process: Weigh the prescribed amount of apigenin, meze and cholesterol in a beaker, add an appropriate amount of ethanol and chloroform mixed solvent to dissolve, evaporate under reduced pressure to remove the organic solvent to form a thin film, add phosphate buffer, hydrate for 1 to 2 Hours, processed by a high-pressure homogenizer for 5 minutes, and passed through a 0.22um filter membrane to obtain an apigenin vesicle solution. The encapsulation efficiency of the obtained vesicles was determined, and the encapsulation efficiency was 68.62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com