Sub-microemulsion preparation for levocarnitine injection and preparation method thereof
A technology for milk preparations and injections, which is applied in the field of medicine, can solve the problems of unstable and unstable product quality, and achieve the effect of simple and easy preparation process, good stability and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
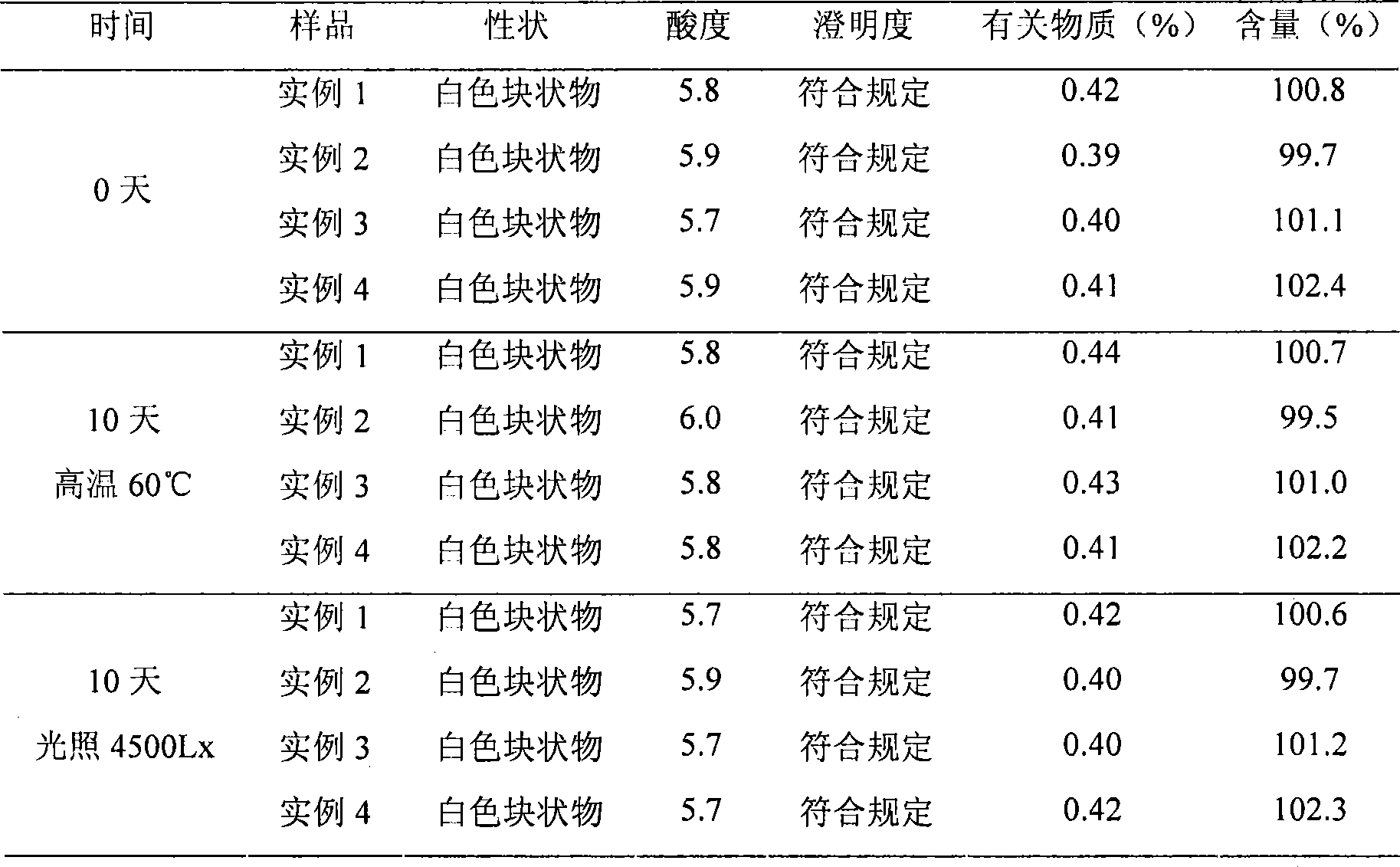
Embodiment 1
[0030] L-Carnitine 500g
[0031] soybean oil 500g
[0032] Soy Lecithin 1000g
[0033] Butylated hydroxytoluene 30g
[0035] Glucose 1000g
[0036] Phosphate buffer solution to pH 5.0-6.5
[0037] Water for injection 5000ml
[0038] Preparation Process:
[0039] (1) Weigh 500g of levocarnitine and evenly disperse it in 500g of soybean oil, add 1000g of soybean lecithin and 30g of dibutyl hydroxytoluene, heat to 50°C, and stir evenly to obtain an oil phase;
[0040] (2) Weigh 1000g of glucose and 20g of sodium bisulfite and dissolve them in water for injection, stir and dissolve to obtain an aqueous phase;
[0041] (3) Mix the prepared oil phase and water phase, heat to 50° C., stir evenly to obtain colostrum, and then further emulsify with a high-pressure homogenizer to obtain levocarnitine emulsion;
[0042] (4) The emulsion was adjusted to 5.8 with phosphate buffer, filtered through a microporous membrane, and freeze-dried to obtain a fr...
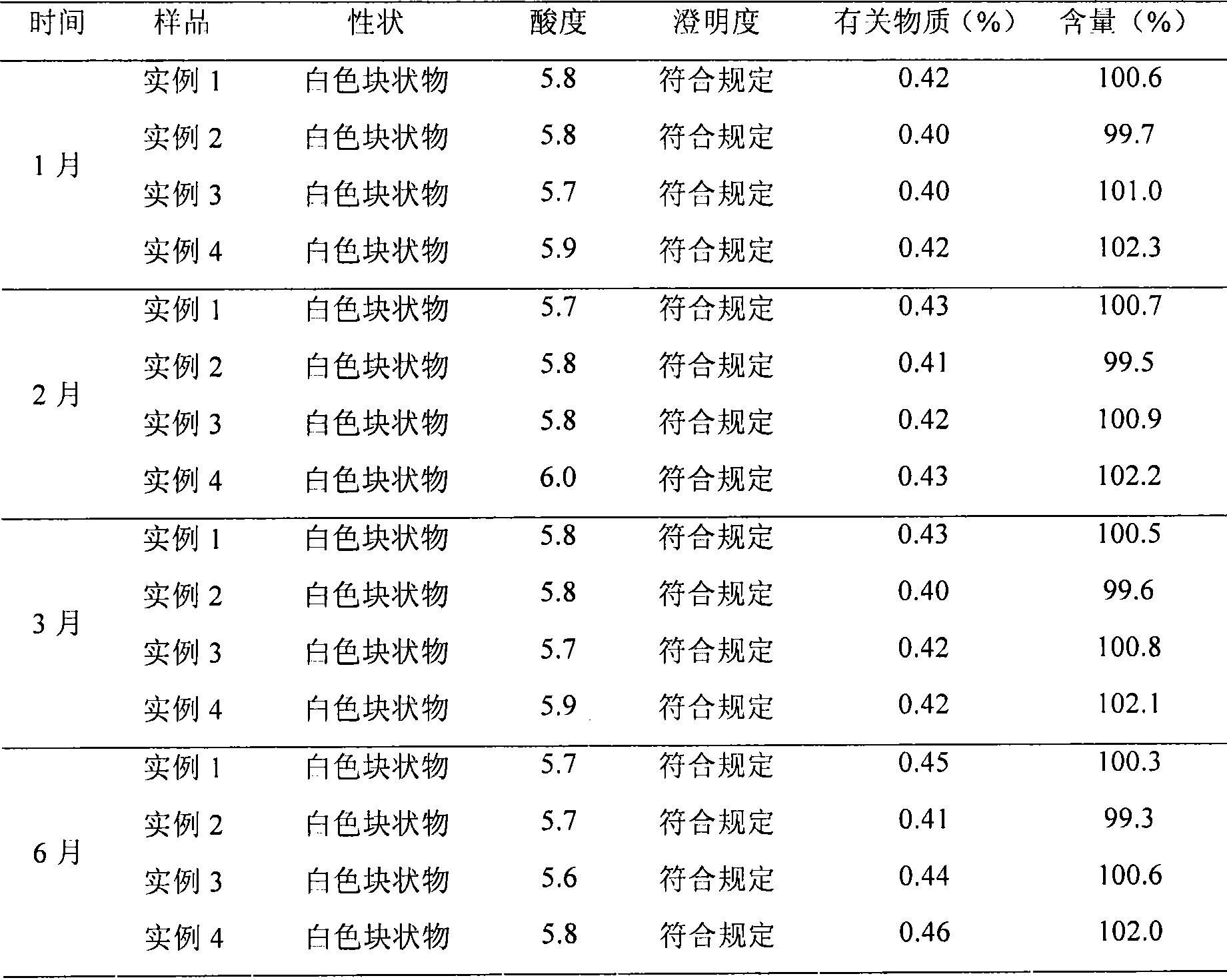
Embodiment 2
[0044] L-Carnitine 1000g
[0045] Peanut oil 3000g
[0046] Vitamin E-Polyethylene Glycol 1000 Succinate 5000g
[0047] Tocopherol 400g
[0048] Ascorbic acid 600g
[0049] Sorbitol 9000g
[0050] Amount of citric acid and sodium citrate buffer to pH 5.0-6.5
[0051] Water for injection 20000ml
[0052] Preparation Process:
[0053] (1) Weigh 1000g of levocarnitine and evenly disperse it in 3000g of peanut oil, add 5000g of vitamin E-polyethylene glycol 1000 succinate and 400g of tocopherol, heat to 60°C, and stir evenly to obtain an oil phase;
[0054] (2) Take 9000g sorbitol and 600g ascorbic acid and dissolve in water for injection, stir and dissolve to obtain the aqueous phase;
[0055] (3) Mix the prepared oil phase and water phase, heat to 60°C, stir evenly to obtain colostrum, and then further emulsify with a high-pressure homogenizer to obtain levocarnitine emulsion;
[0056] (4) The emulsion was adjusted to 5.9 with citric acid and sodium citrate buffer, filtere...
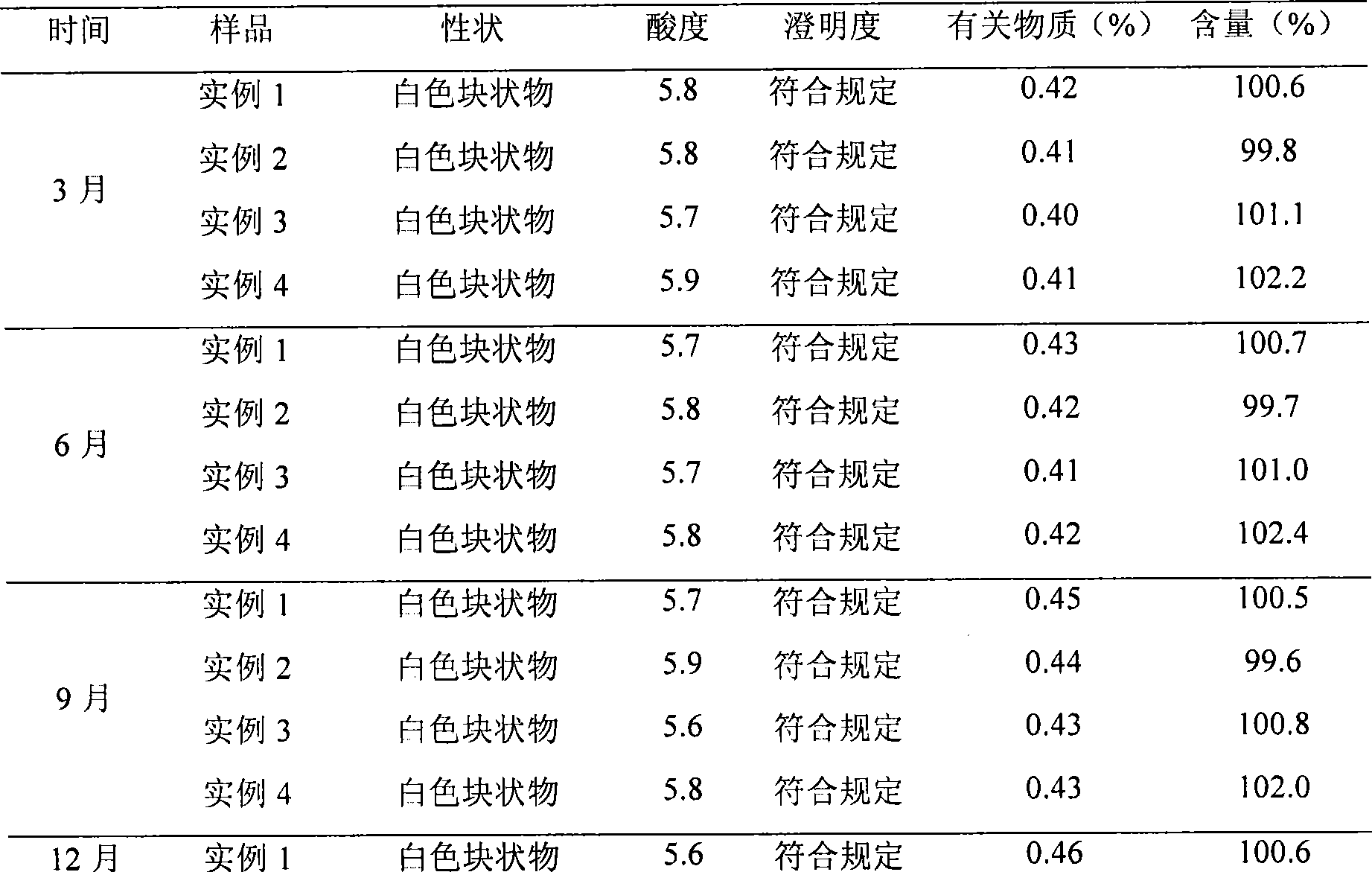
Embodiment 3
[0058] L-Carnitine 500g
[0059] Olive oil 3000g
[0061] Butylated hydroxytoluene 30g
[0062] Ascorbic acid 20g
[0063] Glucose 5000g
[0064] Acetate buffer to pH 5.0-6.5
[0065] Water for injection 10000ml
[0066] The preparation process was the same as that in Example 1, the acetate buffer was adjusted to 5.7, and freeze-dried to obtain a freeze-dried preparation of levocarnitine submicroemulsion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com