Levamlodipine beaylate tablets and preparation method thereof
A technology of levamlodipine besylate and levo-amlodipine besylate, which is applied in the field of levamlodipine besylate tablets and its preparation, can solve the problem of unstable hygroscopicity, small drug specifications, and difficulty in uniform and stable preparations Requirements and other issues, to achieve the effect of improving bioavailability, simple preparation process, and reducing related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
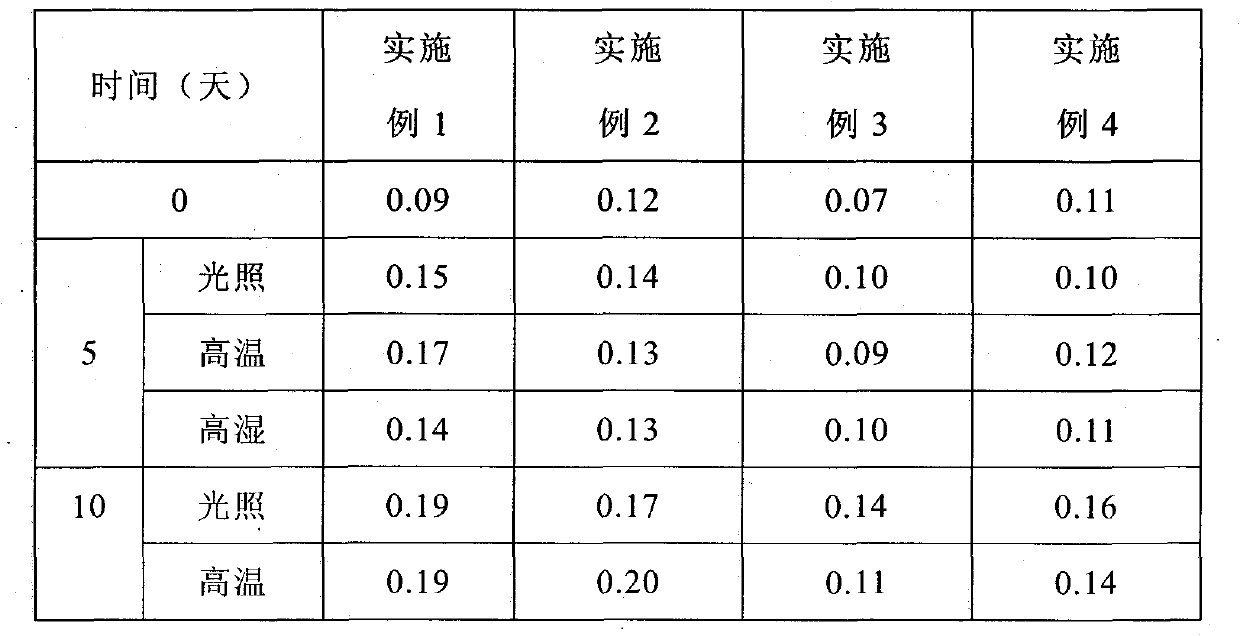
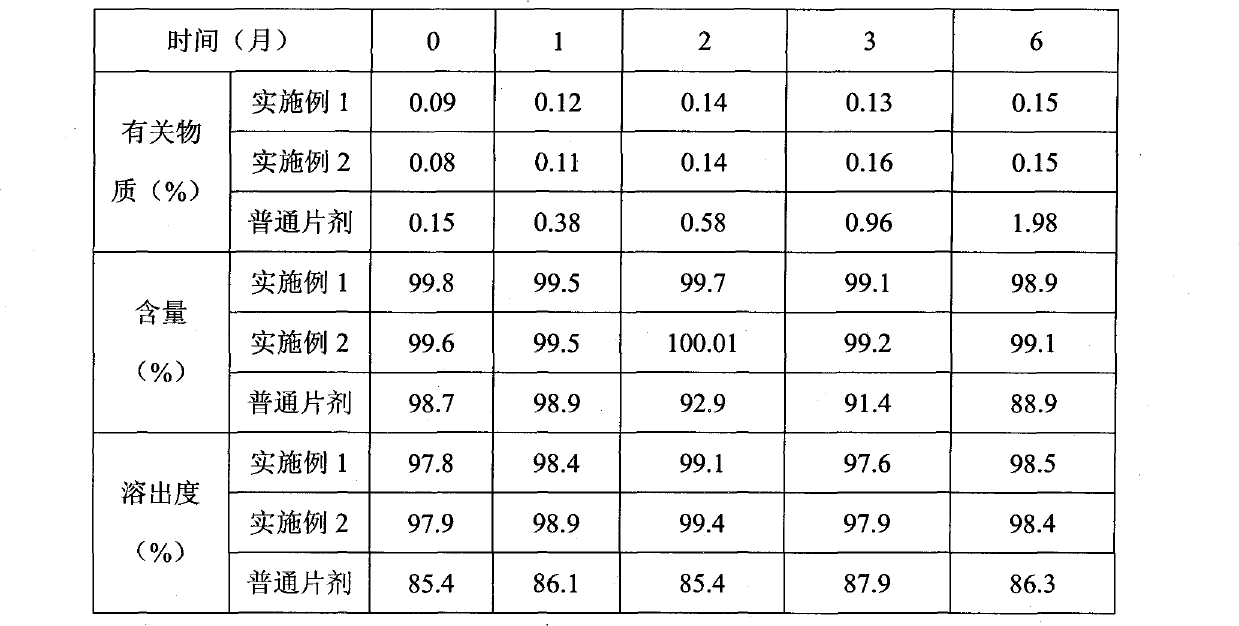
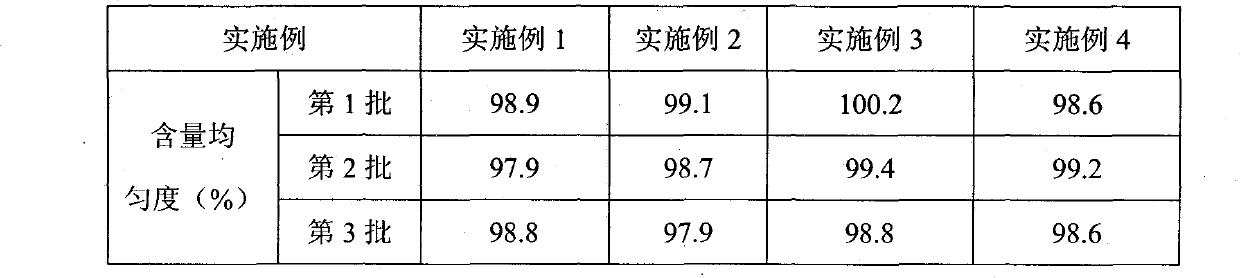
Examples
Embodiment 1
[0033] 1. Prescription composition
[0034] (1) Levoamlodipine besylate tablet core prescription
[0035] Ingredients Dosage
[0036]
[0037] Levoamlodipine Besylate 5g
[0038] Micronized silica gel 20g
[0039]Microcrystalline Cellulose 45g
[0040] Hydroxypropyl Cellulose 10g
[0041] Carboxymethyl Starch Sodium 20g
[0043] 95% ethanol appropriate amount
[0044]
[0045] A total of 1000 pieces were made
[0046] (2) Prescription of coating material
[0047] Ingredients Dosage
[0048]
[0049] Polyvinyl acetal diethylamine acetate 5g
[0050] Hypromellose 1g
[0051] PEG4000 1g
[0053] Titanium dioxide 1.5g
[0054] 5% ethanol 125ml
[0055]
[0056] 2. Preparation method ...
Embodiment 2
[0061] 1. Prescription composition
[0062] (1) Levoamlodipine besylate tablet core prescription
[0063] Ingredients Dosage
[0064]
[0065] Levoamlodipine Besylate 2.5g
[0066] Diatomaceous earth 10g
[0067] Lactose 40g
[0068] Hydroxypropyl Cellulose 30g
[0069] Crospovidone 15g
[0070] Magnesium Stearate 2.54g
[0071]
[0072] A total of 1000 pieces were made
[0073] (2) Prescription of coating material
[0074] Ingredients Dosage
[0075]
[0076] Hypromellose 6g
[0077] PEG6000 1g
[0079] Titanium dioxide 1.5g
[0080] Purified water 100ml
[0081]
[0082] 2. Preparation method
[0083] (1) Weigh the prescription amount of levamlodipine besylate and 2 / 3 of the prescription amount of micropowder silica gel, mix them uniformly by equal volume addition method, the...
Embodiment 3
[0086] 1. Prescription composition
[0087] (1) Levoamlodipine besylate tablet core prescription
[0088] Ingredients Dosage
[0089]
[0090] Levoamlodipine Besylate 5g
[0091] Micronized silica gel 20g
[0092] Pregelatinized starch 50g
[0093] Hydroxypropyl Cellulose 10g
[0094] Croscarmellose Sodium 12g
[0096] 95% ethanol appropriate amount
[0097]
[0098] A total of 1000 pieces were made
[0099] (2) Prescription of coating material
[0100] Ingredients Dosage
[0101]
[0102] Acrylic resin No. IV 8g
[0103] Talc powder 2g
[0104] Titanium dioxide 2g
[0105] 95% ethanol solution 100ml
[0106]
[0107] 2. Preparation method
[0108] (1) Take by weighing the levamlodipine besylate and diatomaceous earth of prescription quantity, after adopting equal am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com