Preparation method of poorly soluble medicine liposome
A technology for insoluble drugs and blank liposomes, applied in the field of pharmaceutical preparation technology, can solve problems such as difficult control, low encapsulation rate, complicated process, etc., and achieve the effect of realizing large-scale industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
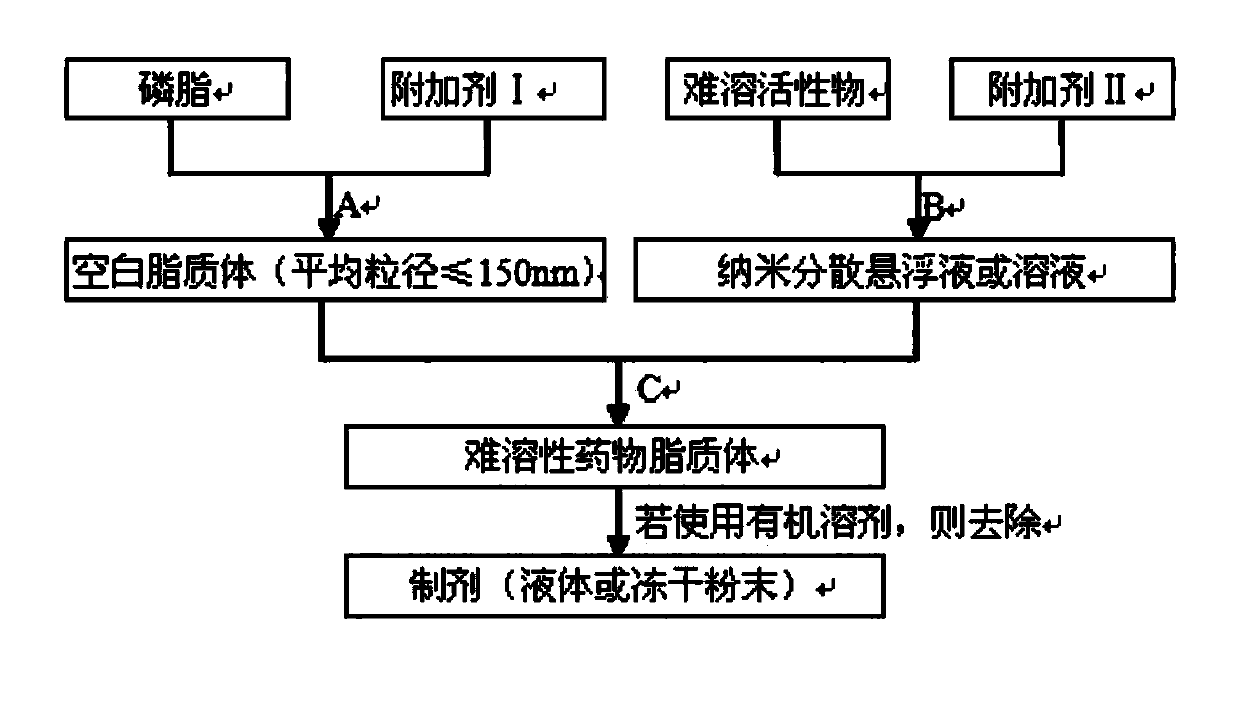
[0050] Preparation Step A
[0051] Blank liposome prescription:
[0052]
[0053] Dissolve phospholipids and soybean oil in chloroform, place on a rotary evaporator, remove chloroform, and form a phospholipid film. Dissolve mannitol in water, add it to the phospholipid film, elute the phospholipid film, and control the average particle size below 150nm through a high-pressure homogenizer for later use.
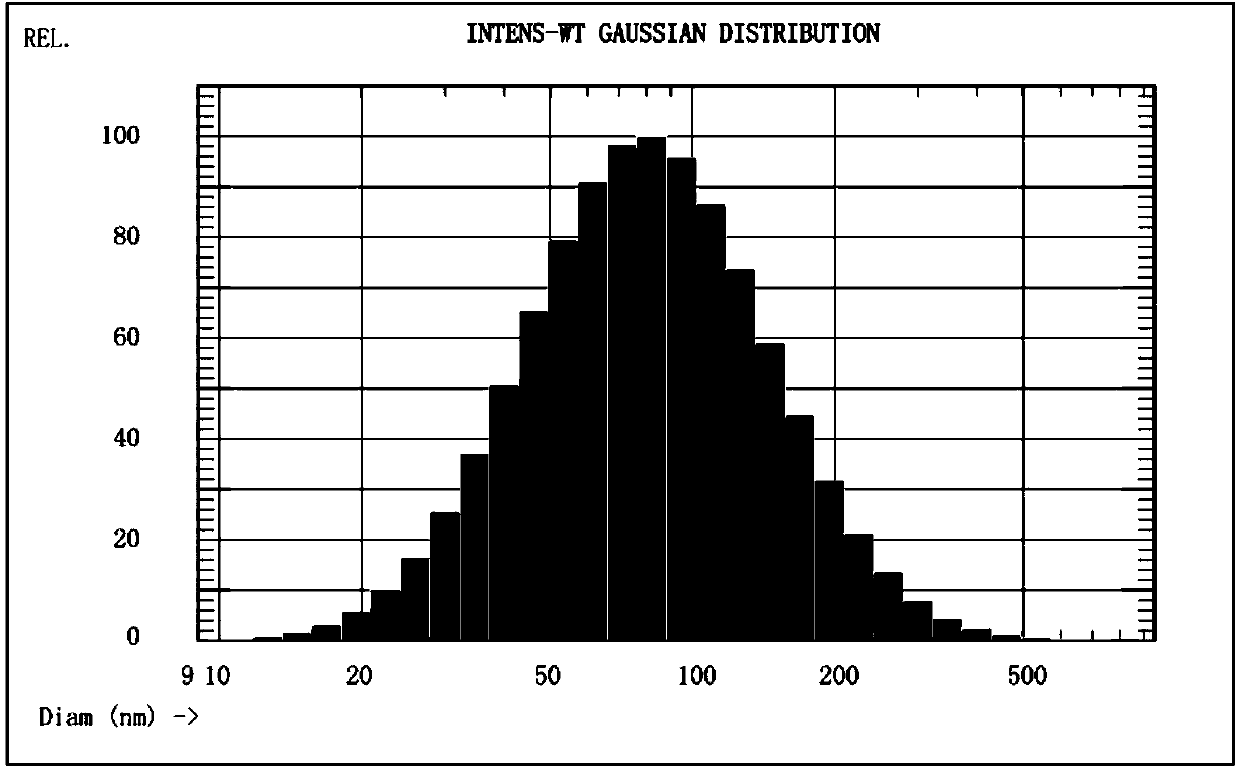
[0054] The particle size chart of the blank liposome is attached figure 2 shown.
[0055] Preparation step B
[0056] Paclitaxel solution prescription:
[0057] Paclitaxel 0.4g
[0058] Ethanol 2g
[0059] Paclitaxel was dissolved in ethanol.
[0060] Preparation Step C
[0061] In step B, add the blank liposome solution prepared in step A (in step B, paclitaxel can be dissolved in ethanol, when the ethanol solution is mixed with water, paclitaxel is insoluble in water and precipitates, and its particles are very small, reaching micron size ), at room temperature,...
Embodiment 2
[0065] Preparation Step A
[0066] Blank liposome prescription:
[0067]
[0068] Heat and dissolve phospholipids, polyethylene glycol phosphatidylethanolamine, cholesterol and ethanol at 60°C, dissolve trehalose and histidine in water, adjust the pH to 6.5 with hydrochloric acid, heat to 65°C, inject the above ethanol solution, and stir for 30 minutes Finally, through a high-pressure homogenizer, the average particle size is controlled below 180nm for later use.
[0069] Preparation step B
[0070] Docetaxel solution prescription:
[0071] Docetaxel 0.6g
[0072] Ethanol 2g
[0073] Dissolve docetaxel in ethanol.
[0074] Preparation Step C
[0075] In step B, add the blank liposome solution prepared in step A (the principle of preparing the insoluble drug suspension or solution is as shown in Example 1), stir at 4°C and 100 rpm, the system turns from turbid to transparent After the buffer solution is replaced by cross tangential flow equipment (the residual amount ...
Embodiment 3
[0079] Preparation Step A
[0080] Blank liposome prescription:
[0081]
[0082] Dissolve phospholipids, phosphatidylglycerol, and cholesterol with methanol and methylene chloride to obtain an organic solution; dissolve sucrose and sodium succinate in water, adjust the pH to 5.5 with hydrochloric acid, and heat to 35°C. Slowly inject the above organic solution, stir for 30 minutes, recover the organic solvent in a vacuum, and then use a high-pressure homogenizer to control the average particle size below 100nm for later use.
[0083] Preparation step B
[0084] Amphotericin B Suspension Prescription:
[0085] Amphotericin B 1g
[0086] Sodium deoxycholate 0.1g
[0087] water 10g
[0088] Sodium deoxycholate was dissolved in water, amphotericin B was added, and ground by a colloid mill so that the average particle size of amphotericin B particles was below 5 μm.
[0089] Preparation Step C
[0090] In step B, add the blank liposome solution prepared in step A, stir a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com