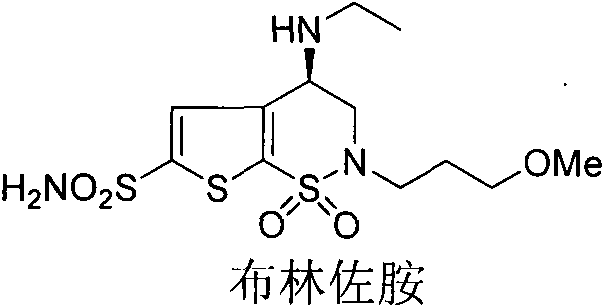
Preparation method of brinzolamide intermediate
A technology of intermediates and compounds, applied in the field of brinzolamide intermediates and its preparation, can solve the problems of harsh reaction conditions, low reaction yield, high risk, etc., and achieve the effect of mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
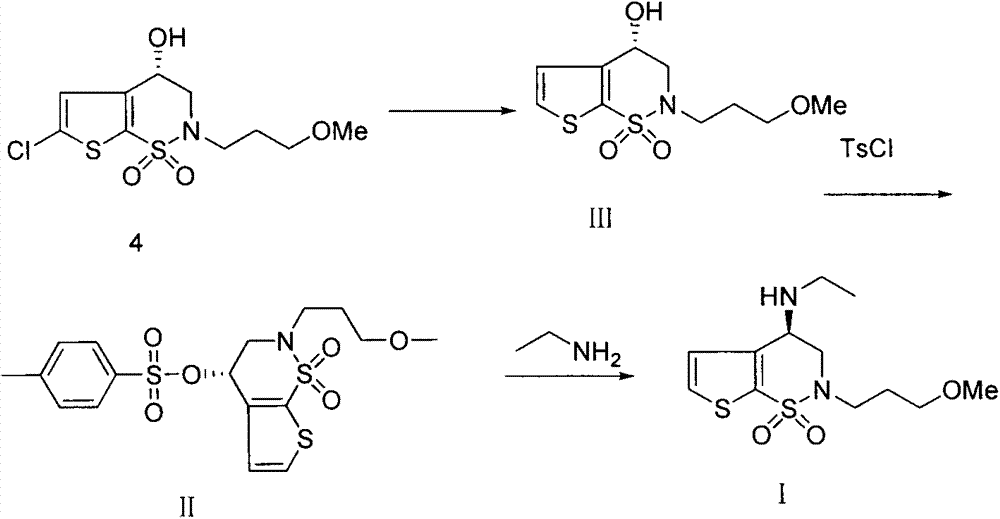
[0029] The preparation of embodiment 1 compound 4
[0030] Add 20g (0.0837mol) of compound 1, 34.6g (0.251mol) of potassium carbonate, and 500ml of DMF solvent into a one-necked flask to dissolve, stir at 70°C for 0.5h, and then add 14.2g of 1-bromo-3-methoxypropane in batches ( 0.09mol), the reaction was continued at 70°C for 2h after the addition. After the reaction was finished, cool naturally, add the reaction solution to 1700ml saturated saline, extract with 2×700ml methyl tert-butyl ether, wash the organic layer with water, then wash with 1300ml sodium hydroxide solution (concentration: 1mol / l) alkali, and then Washed with 3×1.2L saturated brine, finally dried over anhydrous sodium sulfate, and spin-dried at room temperature to obtain 14.2 g of compound 4. 99.23% pure.
[0031] MS: 312.1 ([M+H] + ); 1 HNMR (DMSO) δ6.95(s, 1H), δ4.65(t, 1H), δ4.09(dd, 1H), δ3.80(dd, 1H), δ3.67-3.28(m, 6H) , δ3.25(s, 3H).
Embodiment 2
[0032] The preparation of embodiment 2 compound III
[0033] Weigh 24.2g of compound 4, 6g of palladium on carbon and 200mL of methanol into a single-necked bottle, vacuumize, pass in hydrogen gas to react at room temperature for 12h, after the reaction is completed, add 200ml of ethyl acetate and saturated saline to wash three times after the reaction is completed. Dried over anhydrous sodium sulfate and spin-dried to obtain 22 g of compound III with a purity of 99.68%. MS: 278.1 ([M+H] + ); 1 HNMR (DMSO): δ7.92(d, 1H), δ7.20(d, 1H), δ5.94(d, 1H), δ4.83(m, 1H), δ4.04(dd, 1H), δ3.73 (dd, 1H), δ3.38-3.24 (m, 6H), δ3.22 (s, 3H).
Embodiment 3
[0034] The preparation of embodiment 3 compound II
[0035] Add 22g (0.0794mol) of compound III, 17.6g (0.174mol) of triethylamine and 220ml of tetrahydrofuran into the reaction flask, stir and dissolve at 0°C, add 30.2g of p-toluenesulfonyl chloride (0.159mol), and raise the reaction solution to room temperature and stir After reacting for 18 hours, TLC detected that the reaction was complete, concentrated the reaction solution, added dichloromethane to dissolve, washed the organic phase with water and saturated sodium chloride, dried it with anhydrous sodium sulfate, and concentrated to obtain 27.3 g of compound II, which was directly put into the next experiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com