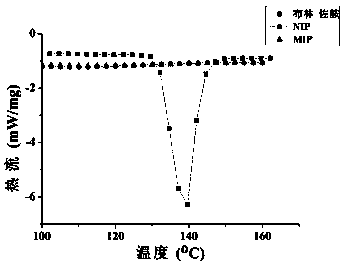
Preparation method of brinzolamide imprinted hydrogel contact lens for sustained and controlled release administration
A contact lens and hydrogel technology, which is applied in the direction of medical formula, glasses/goggles, medical preparations with non-active ingredients, etc., can solve the problems of large side effects and low bioavailability, achieve long residence time and increase flexibility Sex and oxygen permeability, the effect of avoiding environmental pollution and harm to the human body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1. Preparation of Molecularly Imprinted Hydrogel Contact Lens Using Brinzolamide as a Template
[0025] Preparation of DES hydrophilic monomer:
[0026] Choline chloride (3.75 g) and methacrylic acid (4.2 ml) were added to a glass ampoule, and magnetically stirred in an oil bath at 90°C for 24 hours to form a clear and transparent solution, which was stored at room temperature.
[0027] Preparation of pre-polymerization solution:
[0028]Template brinzolamide (0.0455 g), backbone monomer hydroxyethyl methacrylate (1397.5 μL), functional monomer DES (12.9 μL), cross-linking agent polyethylene glycol dimethacrylate (607.5 μL) were added Put it into a glass ampoule, sonicate for 10 minutes until it is uniform and transparent; then add the initiator AIBN (0.0125 g), and treat it with ultrasonication (40 w) at room temperature for 20 minutes to make it dissolve evenly. Afterwards, nitrogen was purged for 10 minutes, and then the solution was left in the dark for 10...
Embodiment 2
[0033] Embodiment 2, the evaluation of hydrogel contact lens loading capacity
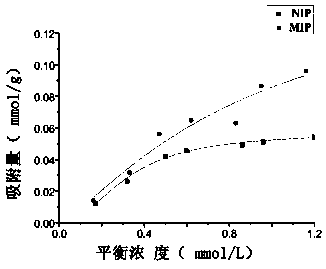
[0034] Carry out according to embodiment 1 step, obtain hydrogel contact lens. Take 10 parts of dried hydrogel lenses, place them in ethanol solution of bulinamide (concentration: 0.1mM-5mM), seal with parafilm, and shake the mixed solution on a constant temperature oscillator for 24h. After equilibrium, centrifuge for 5 minutes in a high-speed centrifuge (5000r / min), take 100 μL of supernatant, and dilute to 10ml with ethanol. The absorbance was then measured with a UV spectrophotometer. according to The adsorption amount was calculated and fitted with the Langmuir-Freundlich model (see image 3 ).
[0035] The detection wavelength is 252nm.
Embodiment 3
[0036] Embodiment 3, the release of brinzolamide drug
[0037] Carry out according to embodiment 1 step, obtain hydrogel contact lens. The obtained lenses were soaked in 100 μg / mL brinzolamide ethanol solution for 3 days, and then washed with distilled water for 5 minutes to remove surface residues. Put the washed lens into a glass bottle containing 10ml of ethanol, put it into the rotor and stir it on a constant temperature magnetic stirrer (rotating speed is 50rp / min), take out 2-3ml from it at regular intervals, and use Measure the absorbance with a UV spectrophotometer (252nm) until there is no significant change in the absorbance. The cumulative release percentage of brinzolamide is plotted against time to obtain the in vitro release curve of the contact lens to the drug (see Figure 4 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com