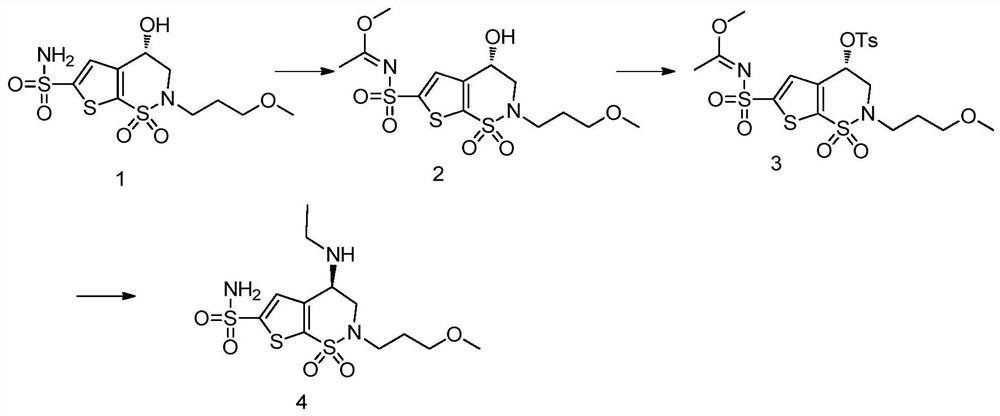
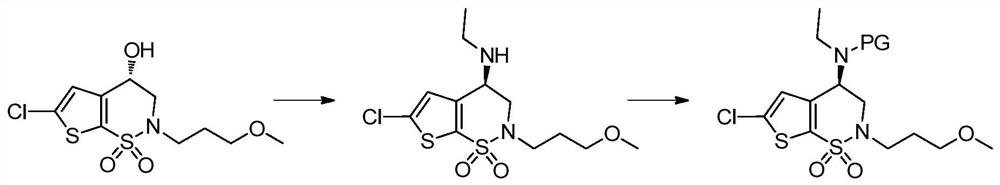
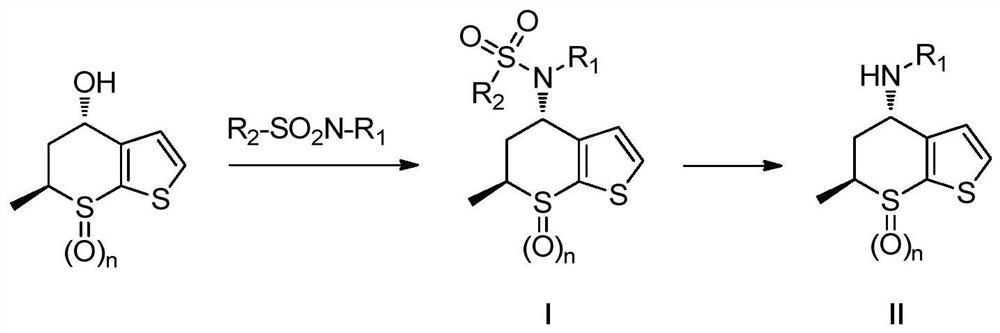
A kind of preparation method of chiral amino compound and its intermediate
A compound and system technology, which is applied in the field of preparation of chiral amine compounds and intermediates thereof, can solve the problems of low reaction yield, bad smell of reaction material benzene mercaptan, elimination of side reactions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] The preparation of embodiment 1 formula B-1 compound
[0083]
[0084] References Organic Process Research & Development1999, 3, 114-120 preparation, specific operations include: prepare a 1L three-neck flask, equipped with a mechanical stirrer, constant pressure low liquid funnel and ice water bath. Add formula A compound (100g, 0.28mol) and acetonitrile (400mL), N 2 Under protection, stirring and cooling to 5-10°C, then trimethyl orthoacetate (77.3 g, 0.64 mol) was added dropwise thereto at a speed to keep the system stable and not exceeding 15°C. After the addition, the cooling was removed, and the reaction was continued for 1 hour. TLC detected that the starting material disappeared, and a new spot with slightly higher polarity was generated. Concentrate under normal pressure, recover the solvent, add DCM to the residue, replace the precipitation with rotary evaporation, and finally dry under high vacuum for 1 hour. The residue is the compound of formula B-1, wh...
Embodiment 2
[0085] The preparation of embodiment 2 formula B-2 compound
[0086]
[0087] The reference document Organic Process Research & Development 1999, 3, 114-120 was prepared, and the specific operations included: preparing a 1L three-neck flask, equipped with a mechanical stirrer, a constant pressure dropping funnel, and an ice-water bath. Added formula A compound (50g, 0.14mol) and acetonitrile (250mL), N 2 Under protection, stirred and cooled to 5-10°C, and then added DMF-DMA (17.5 g, 0.147 mol) dropwise thereto at a speed to keep the system stable and not exceeding 15°C. After the addition, the cooling was removed, and the reaction was continued for 1 hour, and TLC detected that the starting material disappeared. Concentrate under normal pressure, recover the solvent, add DCM to the residue, replace the precipitation with rotary evaporation, and finally dry under high vacuum for 1 hour, the residue is the compound of formula B-2, which can be directly used in subsequent rea...
Embodiment 3
[0088] Example 3 Preparation of Formula C-2 Compound 4-Nitrobenzenesulfonylethylamine
[0089] References Organic Process Research & Development 1999, 3, 114-120 preparation, specific operations include: prepare a 1L there-necked flask, equipped with a mechanical stirrer, add ethylamine aqueous solution (63.5mL, 70%wt%, 0.65mol) and methanol ( 200 mL), under stirring at room temperature, p-nitrobenzenesulfonyl chloride solid (50 g, 0.226 mol) was added in batches. After the addition was complete, the stirring reaction was continued at this temperature for 2 hours, and the reaction was terminated after the TLC detection of the complete disappearance of the raw material. Concentrate under reduced pressure to remove most of the methanol, add 200mL of DCM to dissolve it completely, separate layers, dry the organic phase, concentrate to the remaining 50mL of solvent, add 200mL of n-hexane, stir at room temperature for 30 minutes, filter and dry to obtain white The solid was change...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com