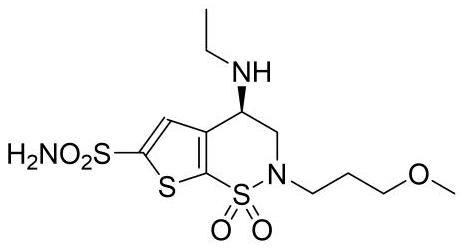
A kind of synthetic method of brinzolamide key intermediate
A synthetic method and intermediate technology, applied in the field of drug synthesis, can solve the problems of high production cost and low production efficiency, and achieve the effects of low production cost, reduced reaction steps and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
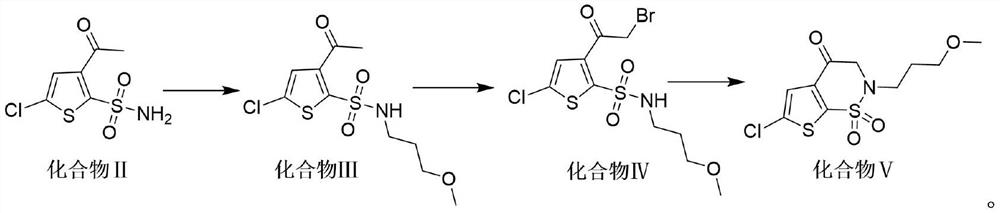
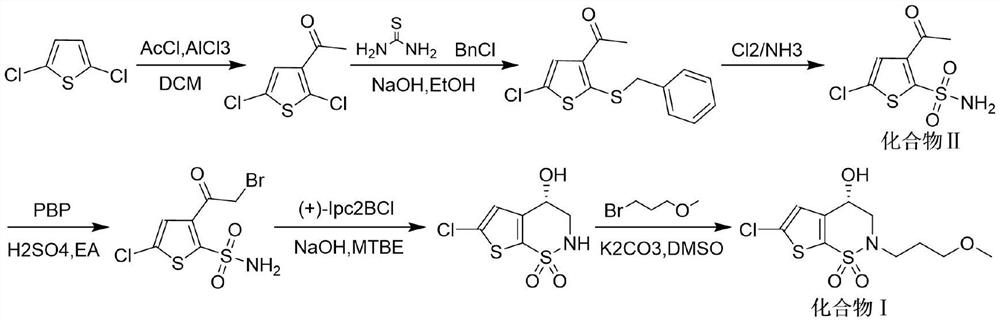
[0036] Preparation of Compound III: Dissolve Compound II 100g (0.42mol) in 2L dimethyl sulfoxide, pour it into a 5L three-necked round-bottomed flask, add 115g (0.83mol) potassium carbonate, 10g (0.04 mol) benzyltriethylammonium chloride and 5g (0.03mol) potassium iodide, then the three-neck round-bottomed flask was heated to 70-80°C, and then 64g (0.6mol) 1-bromo-3-methoxypropane was slowly Drop into a three-necked round-bottomed flask, and the reaction temperature is 70-80°C until the end of the reaction; after the end of the reaction, transfer the product to a separatory funnel, add water and toluene to the separatory funnel, extract, take the upper toluene layer, and pass through After washing, drying, concentration, and adding n-heptane for recrystallization, compound III 111.2 g was obtained, with a yield of 86% and a purity of 98.8% by HPLC.
[0037] HNMR data of compound III: HNMR (DMSO-d 6 ): 8.01 (t, 1H), 7.67 (s, 1H), 3.3-3.2 (m, 4H), 3.15 (s, 3H), 2.57 (s, 3H), 1....
Embodiment 2
[0046] According to the synthetic method in embodiment 1, difference is:
[0047] In the preparation of compound III, dimethyl sulfoxide was replaced by acetonitrile, potassium carbonate was replaced by cesium carbonate of the same molar mass, benzyltriethylammonium chloride was replaced by tetrabutylammonium chloride of the same molar mass, and potassium iodide was replaced by It is sodium iodide with the same molar mass; 1-bromo-3-methoxypropane is 0.5 mol, and the reaction temperature is 70°C to obtain 106.6 g of compound III with a molar recovery of 82.7% and a purity of 98.5% by HPLC.
[0048] HNMR data of compound III: HNMR (DMSO-d 6 ): 8.02(t, 1H), 7.65(s, 1H), 3.3-3.2(m, 4H), 3.13(s, 3H), 2.58(s, 3H), 1.78(m, 2H).
[0049] Mass spectral data of compound Ⅲ: ESI+[M+H] + =312.0
[0050] In the preparation of compound IV, dibromohydantoin was replaced by 0.2 mol of copper bromide, p-toluenesulfonic acid was replaced by 0.01 mol of sulfuric acid (mass fraction is 98%), m...
Embodiment 3
[0057] According to the synthetic method in embodiment 1, difference is:
[0058] In the preparation of compound III, dimethyl sulfoxide was replaced by dimethylformamide of the same volume, potassium carbonate was replaced by sodium methoxide of the same molar mass, and benzyltriethylammonium chloride was replaced by 18- Crown ether-6, potassium iodide was replaced by sodium iodide of the same molar mass, 1-bromo-3-methoxypropane was 0.57mol; the reaction temperature was 80°C, and 104.6g of compound III was obtained, with a molar recovery of 86.1%, HPLC The detection purity is 98.7%.
[0059] HNMR data of compound III: HNMR (DMSO-d 6 ): 8.01 (t, 1H), 7.65 (s, 1H), 3.36-3.21 (m, 4H), 3.13 (s, 3H), 2.57 (s, 3H), 1.78 (m, 2H).
[0060] Mass spectral data of compound Ⅲ: ESI+[M+H] + =312.0
[0061] In the preparation of compound IV, dibromohydantoin was replaced with 0.19 mol of N-bromosuccinimide, p-toluenesulfonic acid was replaced with 0.01 mol of acetic acid, and methanol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com