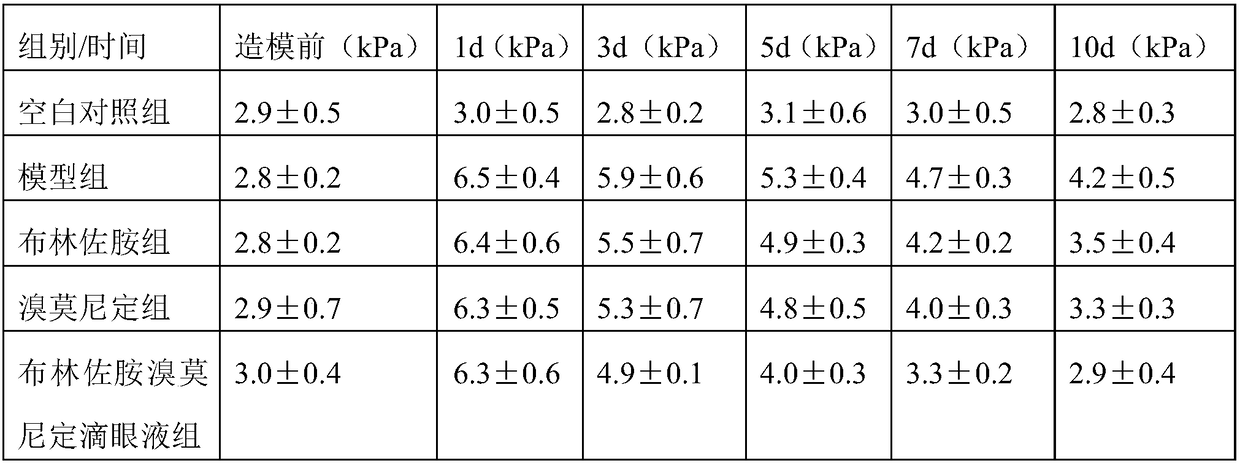
Brinzolamide brimonidine eye drop and preparation method thereof
A technology of brimonidine and eye drops, which is applied in the field of brimonidine brimonidine eye drops and its preparation, can solve the problems of low concentration of N-deethyl-brinzolamide, and promote Corneal epithelial repair, increased comfort, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. a brinzolamide brimonidine eye drop, by weight percentage, it comprises the following components: brinzolamide 2%, brimonidine 2%, sodium chloride 0.5%, potassium chloride 0.3%, 1% sodium hyaluronate and phosphate buffer solution, the balance is water for injection, and the addition amount of the pH regulator is such that the pH of the eye drop is 6.
[0024] 2. a preparation method of brinzolamide brimonidine eye drops, comprising the following steps:
[0025] (1) Take water for injection of 50wt% of the prescription amount, add it to the container together with brinzolamide, brimonidine, pH regulator and osmotic pressure regulator, stir and mix, and use a 0.2 μm filter membrane to filter and sterilize;
[0026] (2) Weigh the amino polysaccharide and add it to the filtrate, and stir at a stirring speed of 150 rpm for 5 hours at a temperature of 20° C. until the amino polysaccharide dissolves;
[0027] (3) Supplement the remaining water for injection, shake well, fi...
Embodiment 2
[0029] 1. a brinzolamide brimonidine eye drop, by weight percentage, it comprises the following components: brinzolamide 1.5%, brimonidine 1.5%, sodium chloride 0.3%, potassium chloride 0.1%, 0.1% chondroitin sulfate and an appropriate amount of sodium hydroxide, the balance is water for injection, and the amount of sodium hydroxide added is such that the pH of the eye drop is 5.5.
[0030] 2. a preparation method of brinzolamide brimonidine eye drops, comprising the following steps:
[0031] (1) Take water for injection of 40wt% of the prescribed amount, add it to the container together with brinzolamide, brimonidine, pH regulator and osmotic pressure regulator, stir and mix, and use a 0.2 μm filter membrane to filter and sterilize;
[0032] (2) Weigh the amino polysaccharide and add it to the filtrate, and stir at a stirring speed of 200 rpm for 6 hours at a temperature of 15°C until the amino polysaccharide dissolves;
[0033] (3) Supplement the remaining water for injecti...
Embodiment 3
[0035] 1. a brinzolamide brimonidine eye drop, by weight percentage, it comprises the following components: brinzolamide 1%, brimonidine 5%, magnesium chloride 0.8%, chitosan 0.5% , 0.5% chondroitin sulfate and an appropriate amount of boric acid, the balance is water for injection, and the amount of boric acid added is such that the pH of the eye drop is 7.5.
[0036] 2. a preparation method of brinzolamide brimonidine eye drops, comprising the following steps:
[0037] (1) Take water for injection of 40wt% of the prescribed amount, add it to the container together with brinzolamide, brimonidine, pH regulator and osmotic pressure regulator, stir and mix, and use a 0.2 μm filter membrane to filter and sterilize;
[0038] (2) Weigh the amino polysaccharide and add it to the filtrate, and stir at a stirring speed of 100 rpm, a stirring time of 3 hours, and a temperature of 25° C. until the amino polysaccharide dissolves;
[0039] (3) Supplement the remaining water for injection...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Osmotic pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com