Preparation methods of brinzolamide and an intermediate thereof
A technology of volume and ethylamine, applied in the field of preparation of brinzolamide and its intermediates, can solve the problems of improved reaction yield and purity, high reaction temperature, cumbersome reaction operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
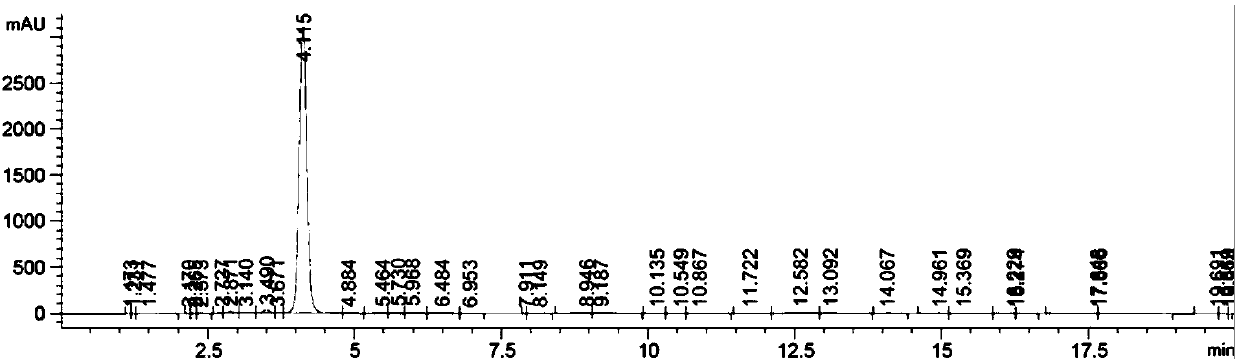
[0108] Preparation of reaction raw materials: Compound B(S)-4-hydroxy-2-(3-methoxypropyl)-3,4-dihydro-thieno[3,2-e]-1,2-thiazine- 6-sulfonamide-1,1-dioxide was prepared by referring to the following synthetic route (reference: Organic Process Research and Development, 1999, 3(2), 114-120).
[0109]
[0110] Compound B NMR data: 1 H NMR (300MHz, DMSO) δ8.04(s, 2H), 7.60(s, 1H), 6.15(d, J=6.0Hz, 1H), 4.83(d, J=5.2Hz, 1H), 3.92(dd ,J=15.3,4.6Hz,1H),3.74(dd,J=15.3,5.6Hz,1H),3.34(dt,J=9.3,6.6Hz,4H),3.23(s,3H),1.83(t, J=6.5Hz, 2H).
Embodiment 1
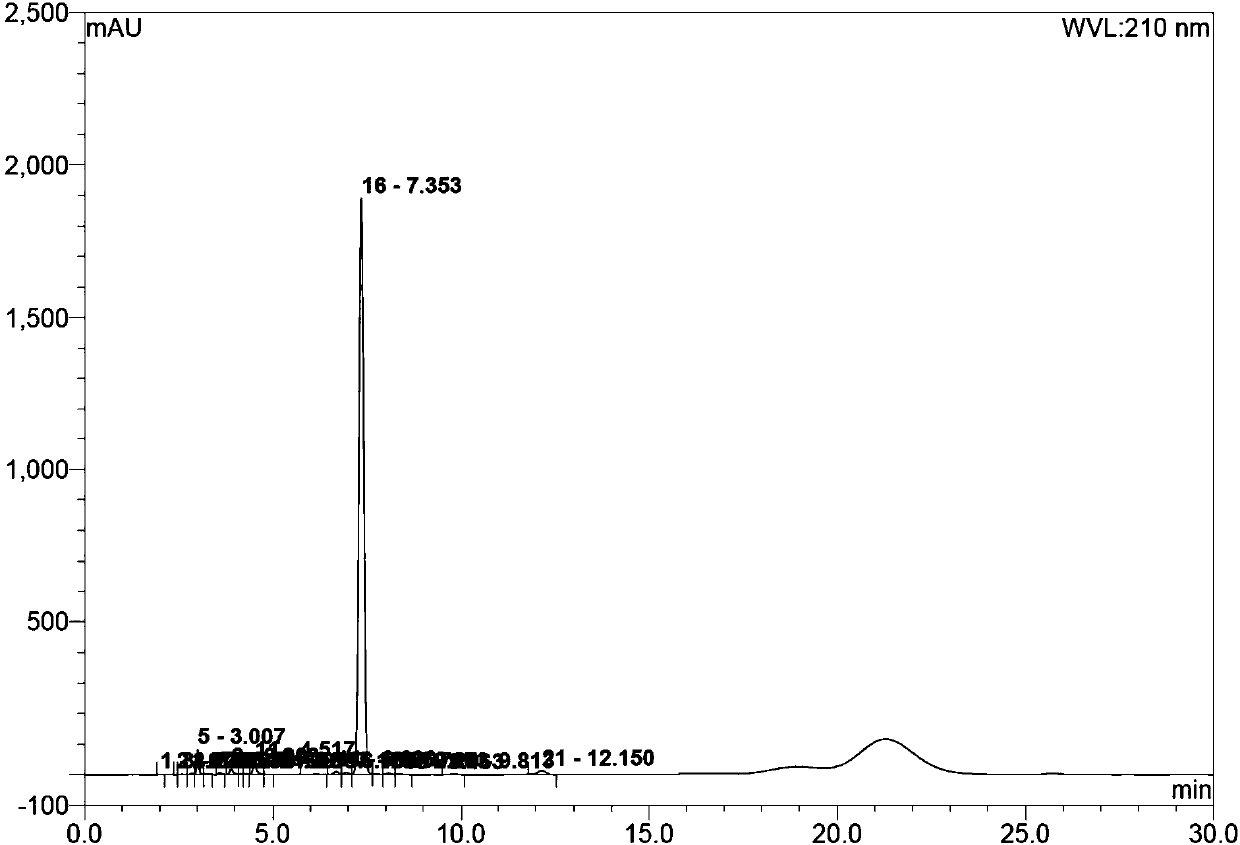
[0112] Compound B (10.0g, 28.1mmol) was added to 90mL of acetonitrile, trimethyl orthoacetate (5.4g, 44.9mmol) and triethylamine (0.28g, 2.8mmol) were added under stirring, and the temperature was raised to 78°C after the addition was completed , stirred for 4h, and HPLC monitoring showed that the reaction was complete. Cool down to 40°C, distill under reduced pressure, and concentrate to the minimum volume to obtain the crude compound C.
[0113] Dissolve the crude compound C in 30mL tetrahydrofuran, cool to -10°C, slowly add triethylamine (6.2g, 61.7mmol) dropwise at a rate of 1d / s; after the addition, add 4- Tosyl chloride (10.7 g, 61.7 mmol) in 20 mL THF. After addition, stir at 5°C, and the reaction is complete in about 3 hours.
[0114] Under controlled temperature of 10°C, 70% ethylamine aqueous solution (72.2 g, 1.12 M) was slowly added dropwise at a rate of 3 d / s. After the addition is complete, the temperature is controlled at 20°C and stirred, and the reaction is...
Embodiment 2
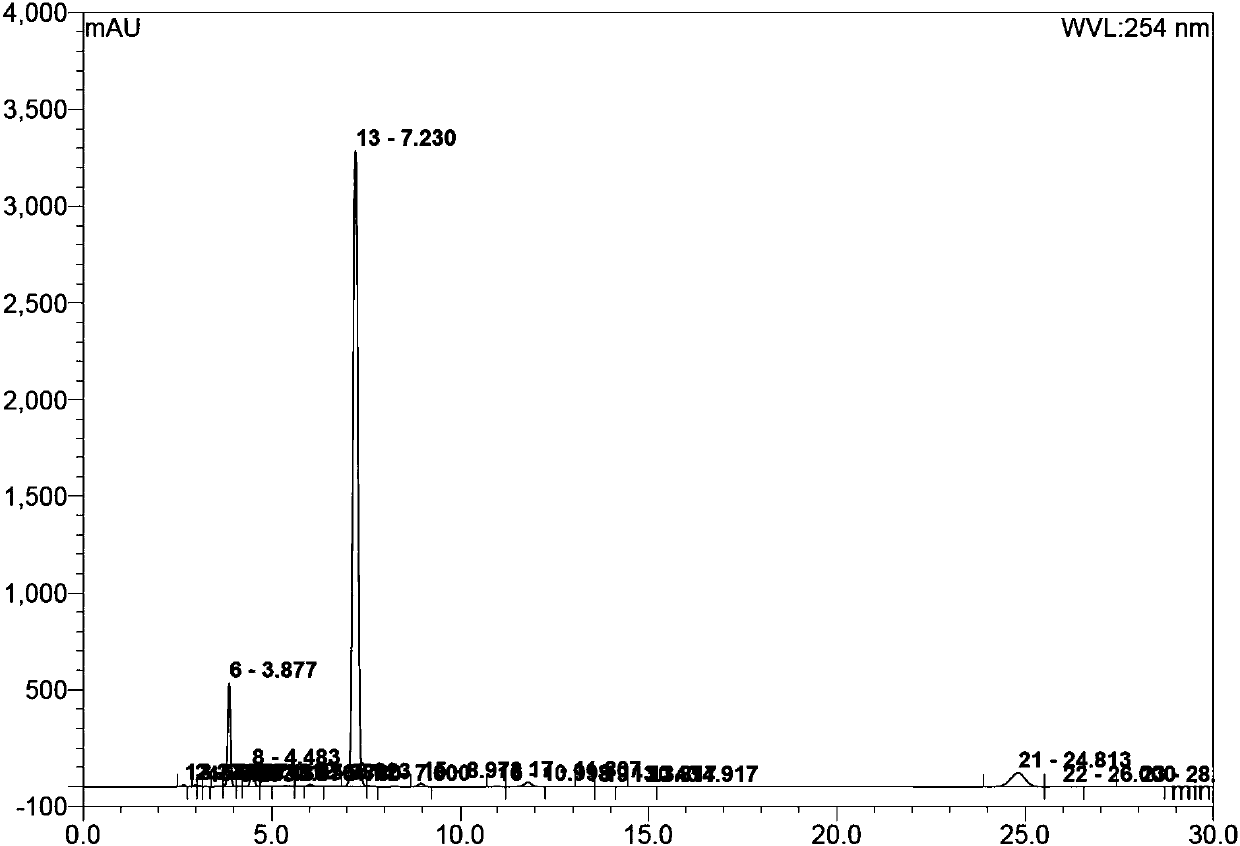
[0120] Compound B (10.0g, 28.1mmol) was added to 90mL of acetonitrile, trimethyl orthoacetate (6.1g, 50.5mmol) and triethylamine (0.28g, 2.8mmol) were added under stirring, and the temperature was raised to 71°C after the addition was completed , stirred for 3h, HPLC monitoring showed that the reaction was complete. Cool down to 32°C, distill under reduced pressure, and concentrate to the minimum volume to obtain the crude compound C.
[0121] Dissolve the crude compound C in 30mL tetrahydrofuran, cool to 0°C, slowly add triethylamine (6.2g, 61.7mmol) dropwise at a rate of 1d / s; after the addition, add 4-toluene dropwise at a rate of 1d / s A solution of sulfonyl chloride (10.7 g, 61.7 mmol) in 20 mL of tetrahydrofuran. After the addition is completed, the temperature is controlled at -5°C and stirred, and the reaction is complete in about 3 hours.
[0122] At a controlled temperature of 5°C, a 70% ethylamine aqueous solution (72.2 g, 1.12 M) was slowly added dropwise at a rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com