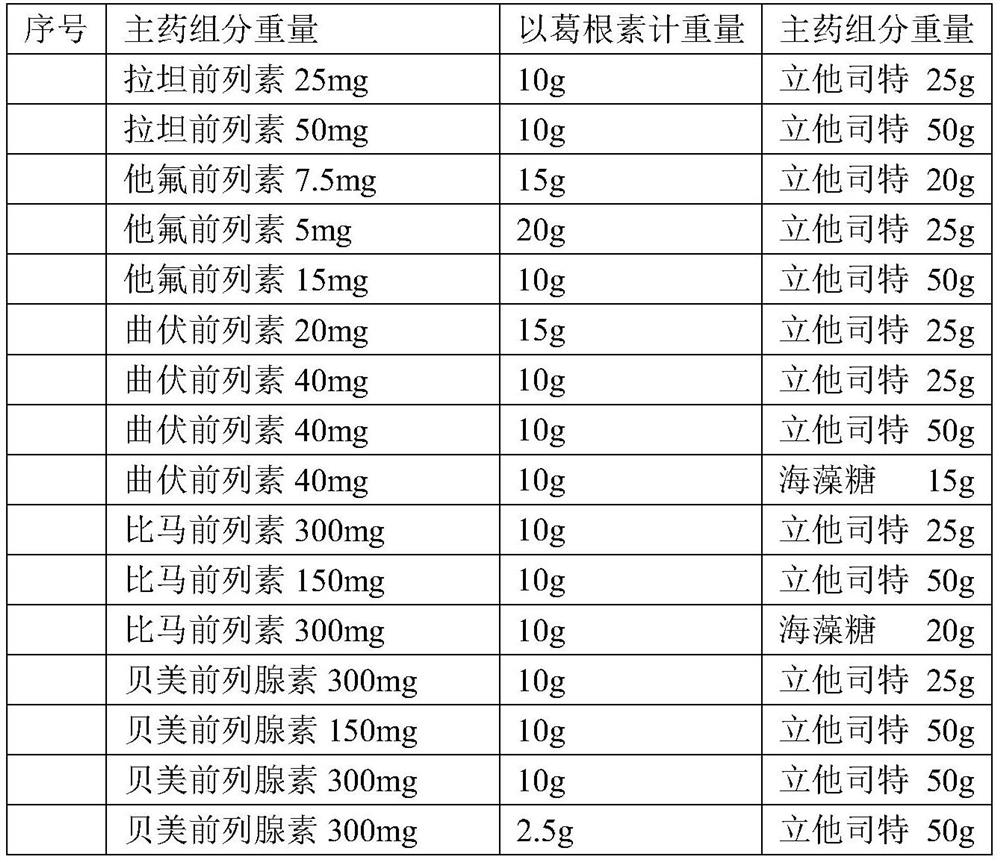
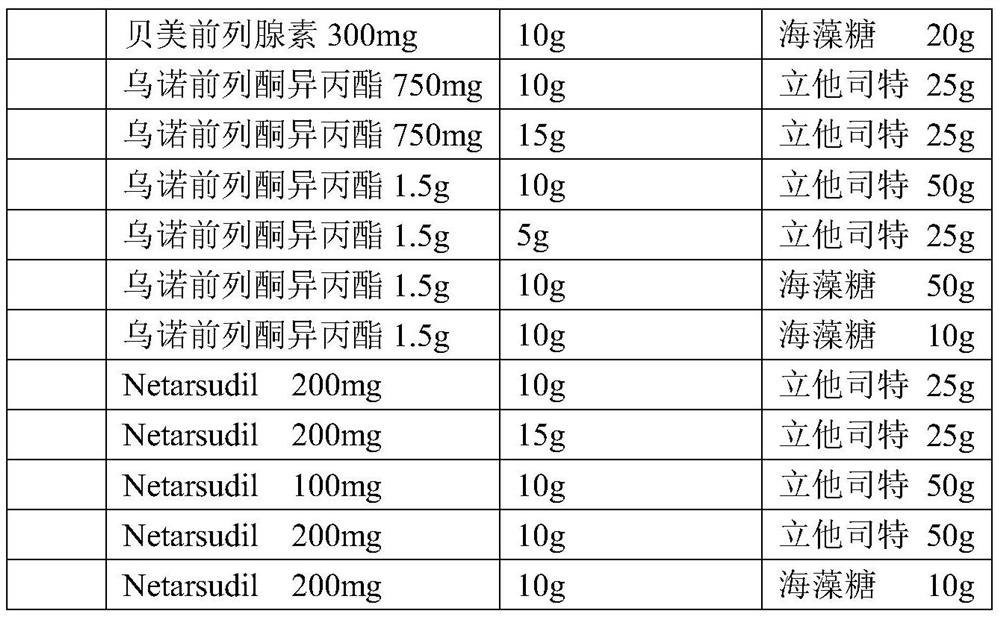
Local-medicine-applying medicine composition including puerarin, brinzolamide and the like
A technology for topical administration and composition, applied in the field of pharmaceutical compositions such as glazolamide for topical administration, can solve the problems of rapid recovery of unfavorable conditions, prolonged treatment time, poor medication compliance, etc., and achieve inhibition or treatment or improvement. The effect of conjunctival hemorrhage symptoms, reducing adverse reactions, and reducing drug costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0112] In the preparation process of each embodiment of the present invention, in the case where the prescription of the embodiment has defined the names of the components, for the sake of simplicity, the names of the components in the prescription can be simplified or omitted. For example, "puerarin monohydrate", "edetate disodium dihydrate", etc., after the prescription components appear, they can be referred to simply as "puerarin", "edetate disodium" and so on.
[0113] Concentration units used in the description of the present invention have molar concentration (M) or (mol / L) or equivalent concentration (N), or percentage concentration etc., time unit can use second (s), minute (min), hour (h) Etc., volume unit can use liter (l or L), milliliter (ml), microliter (μl), mass unit can use gram (g), milligram (mg) and so on.
[0114] The appropriate amount in "appropriate amount of water for injection", "appropriate amount of sodium chloride" and the like all refer to the app...
Embodiment 1
[0118] The preparation of embodiment 1 compound catiolol puerarin in situ gel eye drops
[0119] Prescription: Carteolol hydrochloride 10g, puerarin monohydrate 10g, poloxamer 407 20g, methylcellulose 5g, polyethylene glycol 400 10g, trehalose 10g, glycerin 20g, methionine 3g, edetate di 0.3g of sodium, 0.01g of polyammonium chloride, appropriate amount of 2M citric acid solution and 2M sodium hydroxide solution, and dilute the volume to 1000ml with water for injection.
[0120] Preparation process: Weigh poloxamer 407, glycerin, polyethylene glycol 400, and methylcellulose in the prescribed amount, place them in a beaker of 850ml water for injection, stir to dissolve, and take the prescribed amount of polimonium chloride and hydrochloric acid respectively. Put carteolol, puerarin monohydrate, methionine, and trehalose in a beaker, stir to dissolve, adjust the pH of the solution to 6.8 with an appropriate amount of citric acid solution and sodium hydroxide solution, add water ...
Embodiment 2
[0121] The preparation of embodiment 2 compound dorzolamide puerarin gel eye drops
[0122] Prescription: dorzolamide 20g, puerarin monohydrate 2.5g, carbomer 940 5g, polyvinyl alcohol 12g, mannitol 8g, trehalose 3g, glycerin 5g, sodium sulfobutylbeta cyclodextrin 10g, cattle Sulfonic acid 5g, benzalkonium bromide 0.01g, edetate disodium dihydrate 0.2g, Tween-80 3g, appropriate amount of 2M citric acid solution and 2M sodium hydroxide solution, dilute to 1000g with water for injection.
[0123] Preparation process: Take the prescribed amount of Carbomer 940 and polyvinyl alcohol in a beaker, add about 850ml of water, let it stand still to make it swell, adjust the pH value to about 6.8 with an appropriate amount of citric acid solution and sodium hydroxide, and obtain the matrix. Edetate disodium dihydrate, sulfobutylbeta cyclodextrin sodium, mannitol, glycerin, dorzolamide, puerarin monohydrate, trehalose, taurine, benzalkonium bromide, Add water at temperature -80 and stir ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com