Method for purifying tafluprost
A technology of tafluprost and purification method, which is applied in chemical instruments and methods, organic chemistry, pharmaceutical formulations, etc., and can solve problems such as low yield, long manufacturing process, and low practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0095] Hereinafter, reference examples, examples, and test examples are given to describe the present invention in detail, but the present invention is not limited thereto.
[0096] % represents mol% in yield, and represents mass% unless otherwise specified. The ratio shown in the mixed solvent means a volume ratio unless otherwise specified. In addition, room temperature means a temperature of 15 to 30°C unless otherwise specified. below 1 The H-NMR value was measured with a nuclear magnetic resonance apparatus ECP400 (400 MHz) manufactured by JEOL Ltd. HPLC apparatus used Shimadzu LC-10ADvp or LC-10A. As a GC apparatus, Shimadzu GC-2014ATF was used.
reference example 1
[0097] Reference Example 1: Synthesis of Tafluprost Acid
[0098]
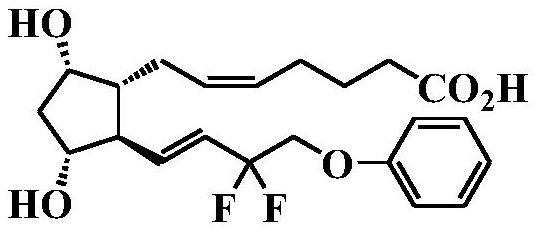
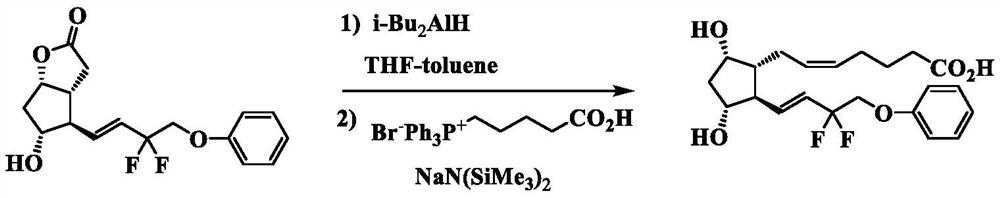
[0099] Under nitrogen atmosphere, in (1S,5R,6R,7R)-6-[(1E)-3,3-difluoro-4-phenoxy-1-butenyl]-7-hydroxyl-2-oxo Tetrahydrofuran (1200 g) was added to heterobicyclo[3.3.0]octan-3-one (280 g) for dissolution, and diisobutylaluminum hydride (1M toluene solution) (2160 mL) was added dropwise at -70°C. After completion of the dropwise addition, the mixture was stirred for 30 minutes, 1N hydrochloric acid was added, and extraction was performed with ethyl acetate. After the organic layers were combined and washed with water, the filtrate was concentrated under reduced pressure to obtain a reduced product (284 g). Under a nitrogen atmosphere, tetrahydrofuran (5030 g) was added to 4-carboxybutyltriphenylphosphonium bromide (1523 g), and sodium bis(trimethylsilyl)amide solution (1M tetrahydrofuran solution) (6684 mL) was added dropwise, Stir for 1 more hour. The above-mentioned reduced product (286 g) dissolved in ...
reference example 2
[0101] Reference example 2: Synthesis of tafluprost crude product
[0102]
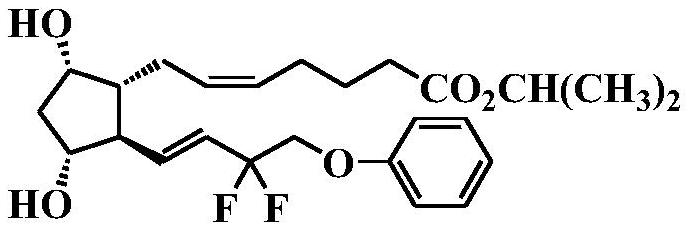
[0103] Under a nitrogen atmosphere, the tafluprost acid (120 g) obtained in Reference Example 1 was charged into a 5 L flask, and dissolved in acetone (600 mL) while stirring. Cool to 5°C, add 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (160mL) dropwise while keeping below 5°C, and then drop while keeping below 5°C After adding 2-iodopropane (146 mL), it was stirred at 30°C until the conversion rate of the reaction was above 95%. Ethyl acetate (1800 mL) and 5% citric acid aqueous solution (900 mL) were added to the reaction mixture for liquid separation, and 5% citric acid aqueous solution (900 mL, once), 5% sodium bicarbonate aqueous solution (900 mL, twice), and purified water (900 mL, once) to wash the organic layer. Under reduced pressure, the solvent was distilled off below 40° C., thereby obtaining a crude product of tafluprost (132 g, yield 100%; HPLC purity: 95.5%, α-chain trans-isomer conte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com