The preparation method of tafluprost
A technology of tafluprost and compound, applied in the field of pharmacy, can solve the problems of high content of excessive reduction impurities, high content of trans-isomer impurities, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
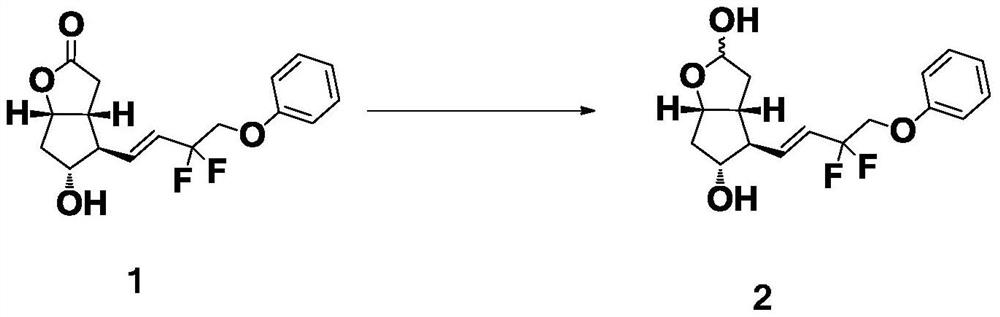
[0034] The preparation of formula 2 compound
[0035] Add 50.0 g of the compound of formula 1 into a four-necked reaction flask filled with 500 mL of anhydrous tetrahydrofuran, and lower the temperature to -70°C±10°C. Under nitrogen protection, DIBAL-H solution (245.6g, 2.8eq) was slowly added dropwise. After dropping, keep the temperature and continue to stir the reaction for 1h, keep at -70°C±10°C, slowly add methanol (9.45g, 8.0eq) dropwise, stir for 0.5h, and quench the reaction. The above mixture was added to 1000 mL of water, and then 1000 mL of ethyl acetate was added, and stirred overnight until the organic phase became clear. Extract and separate the liquids, combine the organic phases; wash the organic phase with saturated brine, let stand to separate the liquids; add silica gel to the organic phase to mix the sample, and separate by column chromatography: prepare n-heptane / triethylamine (17.0kg / 36.5g) Punch the column; the receiving bucket collects 4L each time. ...
Embodiment 2
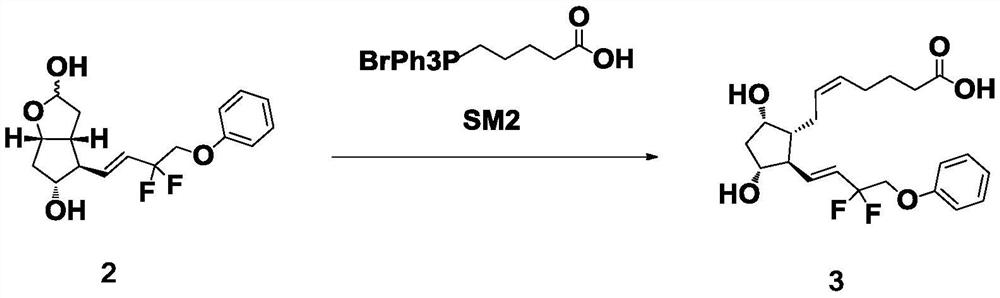
[0037] The preparation of formula 3 compound
[0038] Add 33.0 g of the compound of formula 2 into a four-necked reaction flask filled with 500 mL of anhydrous tetrahydrofuran, cool down to 5±5° C., and add NaHMDS solution (359.7 g, 8.0 eq) dropwise under nitrogen protection. After dropping, keep the temperature at 5°C±5°C and continue stirring for 1h. The temperature was lowered to -20±5°C, and 160 mL of a tetrahydrofuran solution of 33.0 g of the compound of formula SM2 was slowly added dropwise into the system. After dropping, the temperature of the reaction solution was raised to 0±5°C and stirred for 1 h. The reaction solution was poured into 495 g of ice-water mixture, stirred to quench the reaction, 330 mL of ethyl acetate was added, and stirred for liquid separation. The organic phase was extracted once with water, and the aqueous phases were combined; the aqueous phase was extracted twice with ethyl acetate, and the organic phase was discarded. Add 330 mL of ethyl ...
Embodiment 3
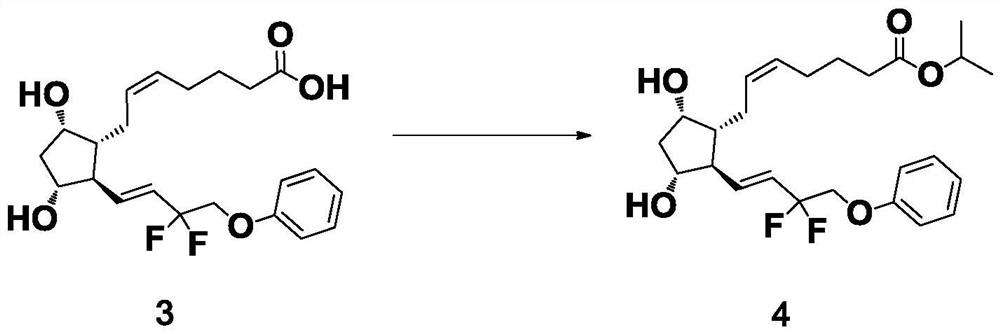
[0040] The preparation of formula 4 compound
[0041] 16.99 g of 1,8-diazabicycloundec-7-ene and 18.99 g of 2-iodopropane were successively added to an acetone solution of 33.0 g of the compound of formula 2, heated under reflux at 55±5°C and stirred. The reaction solution was cooled to room temperature, 460 mL of dichloromethane and 138 mL of water were added, and stirred to dissolve. Extract and separate, and keep the organic phase. Concentrate the organic phase under reduced pressure to a small volume, add silica gel to mix the sample to make sand, and separate by column chromatography. The column fractions of the samples were collected, combined and concentrated to obtain 35.0 g of the crude product as a light yellow oily liquid, with a purity of 99.4%, a trans isomer content of 0.34%, and a yield of 93.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com