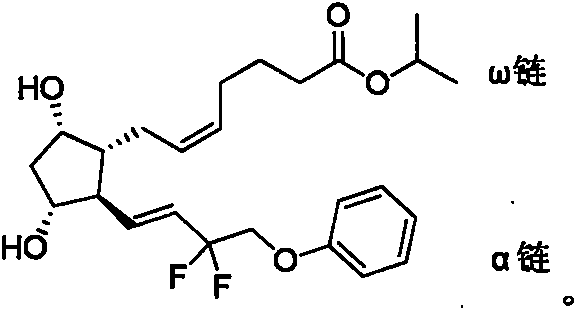
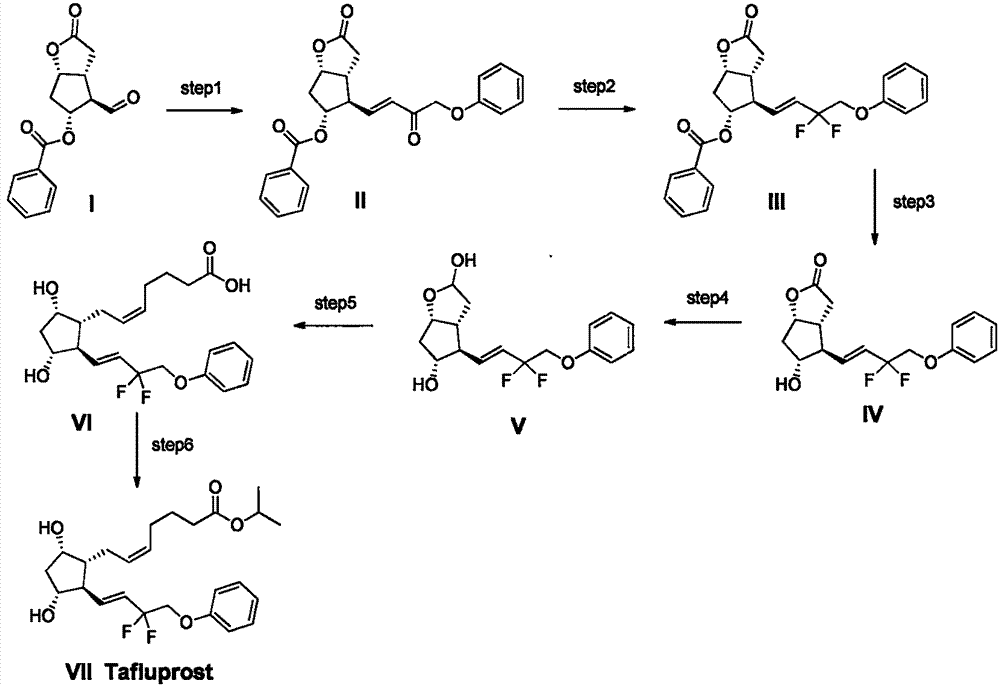
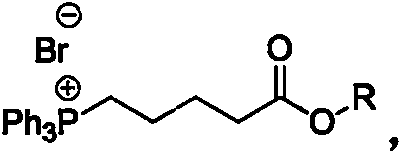
Preparation method of high-purity Tafluprost and analogs thereof and intermediate compound
A technology of tafluprost and compounds, applied in the field of medicine, can solve problems such as not being suitable for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: 5-(triphenylphosphine) ethyl n-pentanoate bromide
[0029] Ethyl 5-bromo-n-valerate (4.16g) and triphenylphosphine (5.24g, 1eq) were dissolved in 100mL of anhydrous toluene and refluxed for 2 days under N2 protection. Cool down to room temperature, filter the precipitated white solid, and rinse with cold n-hexane. The resulting solid was dried in vacuo to obtain 8.46 g of the title compound (90% yield)
Embodiment 2
[0030] Embodiment 2: 5-(triphenylphosphine) isopropyl bromide n-pentanoate
[0031] Isopropyl 5-bromo-n-valerate (4.44 g) and triphenylphosphine (5.24 g, 1 eq) were dissolved in 100 mL of anhydrous toluene and refluxed for 2 days under N2 protection. Cool down to room temperature, filter the precipitated white solid, and rinse with cold n-hexane. The resulting solid was dried in vacuo to obtain 9.0 g of the title compound (93% yield)
[0032] HNMR: 7.23~7.56(15H), 3.89(1H), 2.32~2.87(4H), 1.45~1.75(4H), 1.32(6H)
Embodiment 3
[0033] Example 3: 5-(triphenylphosphine) tert-butyl n-pentanoate bromide
[0034] tert-butyl 5-bromo-n-valerate (4.72g) and triphenylphosphine (5.24g, 1eq) were dissolved in 100mL of anhydrous toluene and refluxed for 2 days under N2 protection. Cool down to room temperature, filter the precipitated white solid, and rinse with cold n-hexane. The resulting solid was dried in vacuo to obtain 7.1 g of the title compound (yield 71%)
[0035] HNMR: 7.23~7.56(15H), 2.32~2.87(4H), 1.45~1.75(4H), 1.25(9H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com