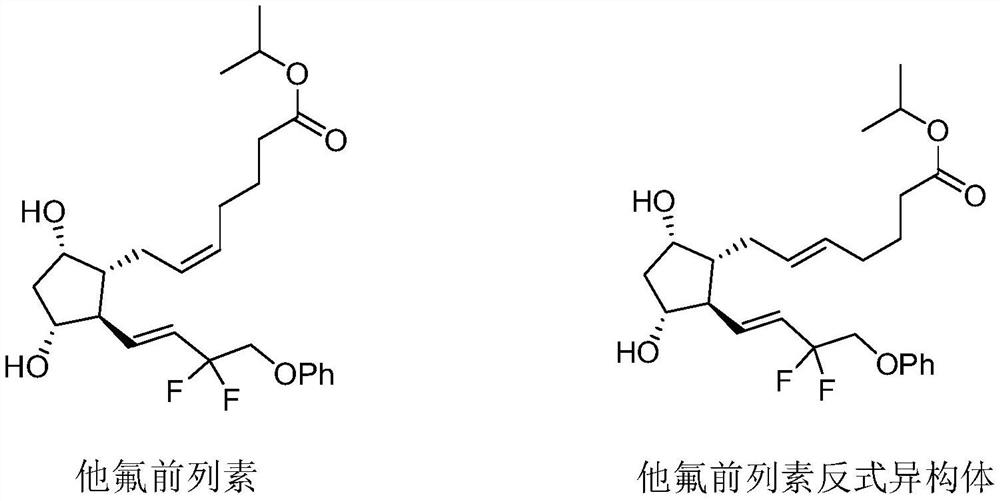
A kind of preparation method of tafluprost crude drug
A technology of tafluprost and raw material medicine, which is applied in the field of pharmacy, can solve the problems of many refining times, low yield, high price, etc., and achieve the effect of high product purity, simple operation and strong repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1, Example 2 (directly proceed to Example 3) and Example 3 without using the subsequent L-arginine salt-forming and refining process, but are not limited to this method.
[0045] In the following examples, unless otherwise specified, all are common commercially available products.
[0046] Example 1
[0047] Preparation and separation of high-efficiency preparative liquid phases
[0048] Preparation of diluent: Add 20ml of isopropanol into 80ml of n-hexane at room temperature, stir to make it evenly mixed.
[0049] Sample preparation: take 25 g of crude tafluprost with a purity of 90% (trans isomer content 0.81%), add 100 ml of diluent, dissolve the sample at room temperature, and filter with a 0.22 μM filter membrane.
[0050] Prepare the mobile phase: add 15 L of isopropanol to 85 L of n-hexane, stir evenly, and filter with a 0.22 μM filter membrane.
[0051] Preparation method: The total running time of each needle sample is 13min, the detection wavelength...
Embodiment 2
[0055] Preparation and separation of high-efficiency preparative liquid phases
[0056] Preparation of diluent: Add 15ml of isopropanol into 85ml of n-hexane at room temperature, stir to make it evenly mixed.
[0057] Sample preparation: take 10 g of crude tafluprost with a purity of 90% (trans isomer content 1.37%), add 70 ml of diluent, dissolve the sample at room temperature, and filter with a 0.22 μM filter membrane.
[0058] Prepare the mobile phase: add 6L of isopropanol to 34L of n-hexane, stir evenly, and filter with a 0.22 μM filter membrane.
[0059] Preparation method: The total running time of each needle sample is 13min, the detection wavelength is 254nm, and the temperature is 25±5°C. The chromatographic column is a Unichiral OZ-5H normal phase preparative column, 50×250 mm, 5 μM. Each injection volume is 6mL, and the main peak height of the automatic collection is greater than 100mAU. The collected product solutions are combined, concentrated at 40±5°C, and th...
Embodiment 3
[0063] Preparation and separation of high-efficiency preparative liquid phases
[0064] Preparation of diluent: Add 20ml of isopropanol into 80ml of n-hexane at room temperature, stir to make it evenly mixed.
[0065] Sample preparation: take 14.5 g of crude tafluprost with a purity of 90% (0.45% trans-isomer content), add 58 ml of diluent, dissolve the sample at room temperature, and filter with a 0.22 μM filter membrane.
[0066] Prepare the mobile phase: add 9 L of isopropanol to 53 L of n-hexane, stir well, and filter with a 0.22 μM filter membrane.
[0067] Preparation method: The total running time of each needle sample is 13min, the detection wavelength is 254nm, and the temperature is 25±5°C. The chromatographic column is a Unichiral OZ-5H normal phase preparative column, 50×250 mm, 5 μM. Each injection volume is 4.5mL, the main peak height is automatically collected and the peak height is greater than 100mAU, the collected product solutions are combined, concentrate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com