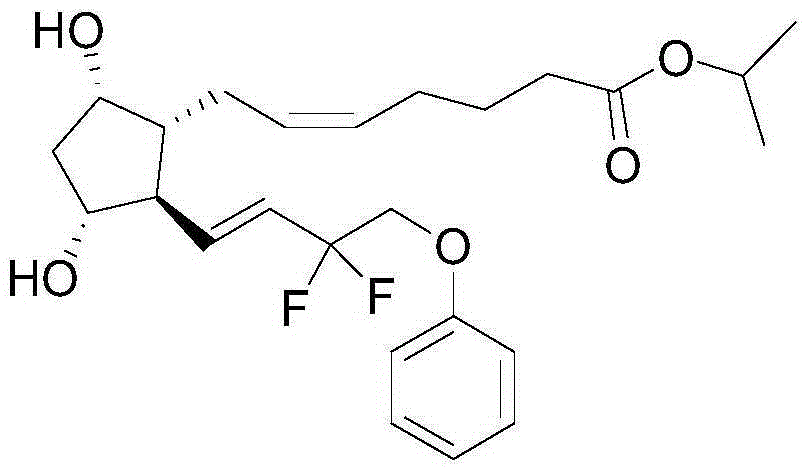
Tafluprost eye drop and preparation method thereof
A technology of tafluprost and eye drops, applied in the field of medicine, can solve the problems of poor water solubility, poor stability and low solubility of tafluprost, and achieve the effects of improving curative effect, reasonable preparation process and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
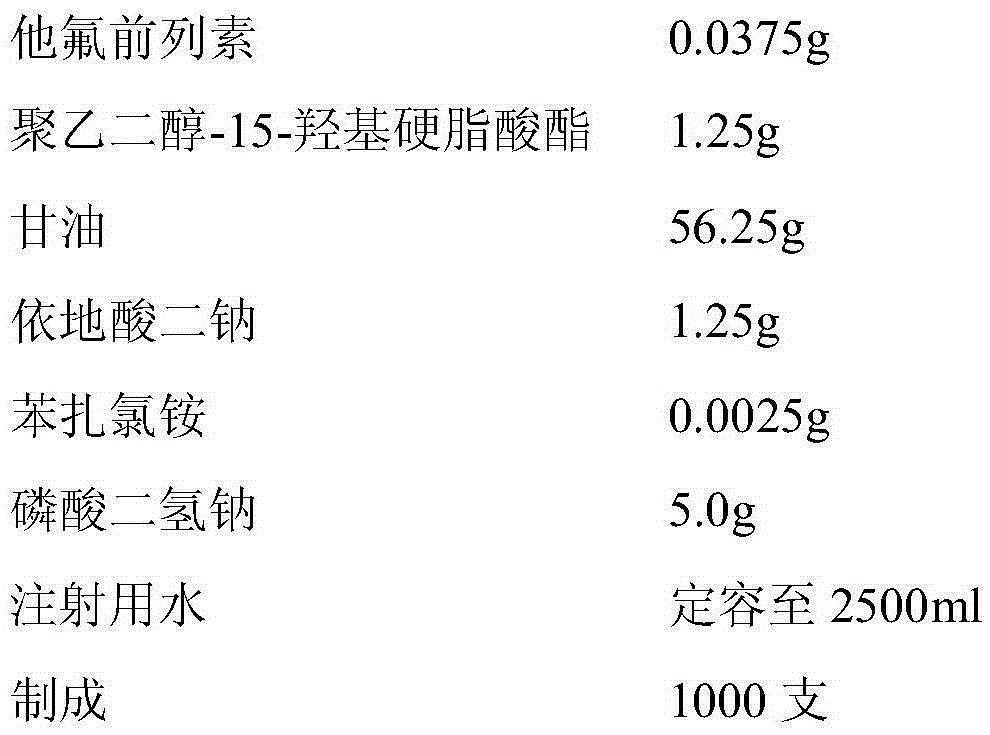
[0028] prescription:
[0029]
[0030] Preparation Process:
[0031] Weigh polyethylene glycol-15-hydroxystearate and dissolve in water for injection, add tafluprost, stir to dissolve; take glycerin, EDTA-2Na, benzalkonium chloride, disodium hydrogen phosphate and dissolve in boiling In water for injection, stir to dissolve; after cooling to room temperature, combine the two liquids, filter, dilute with water for injection to prepare the full amount, adjust the pH value to 6.0, filter through a 0.22μm microporous membrane, and aseptically fill in plastic eye drops In a water bottle, that is.
Embodiment 2
[0033] prescription:
[0034]
[0035]
[0036] Preparation Process:
[0037] Weigh polyethylene glycol-15-hydroxystearate and dissolve in water for injection, add tafluprost, stir to dissolve; take sodium chloride, EDTA-2Na, benzalkonium chloride, disodium hydrogen phosphate and dissolve in In boiling water for injection, stir to dissolve; after cooling to room temperature, combine the two liquids, filter, dilute with water for injection to prepare the full amount, adjust the pH value to 6.0, filter through a 0.22μm microporous membrane, and aseptically fill in plastic Available in eye drops bottle.
Embodiment 3
[0039] prescription:
[0040]
[0041] Preparation Process:
[0042] Weigh polyethylene glycol-15-hydroxystearate and dissolve in water for injection, add tafluprost, stir to dissolve; take glycerin, EDTA-2Na, ethyl p-hydroxybenzoate, disodium hydrogen phosphate and dissolve in In boiling water for injection, stir to dissolve; after cooling to room temperature, combine the two liquids, filter, dilute with water for injection to prepare the full amount, adjust the pH value to 6.0, filter through a 0.22μm microporous membrane, and aseptically fill in plastic Available in eye drops bottle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com