Good-stability eye-drop preparation containing PGF2alpha derivative and preparation method thereof
A technology for ophthalmic preparations with good stability, which is applied in the field of ophthalmic preparations for lowering intraocular pressure and its preparation, and can solve the problems of reduced dosage, poor water solubility, and high biological activity of tafluprost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
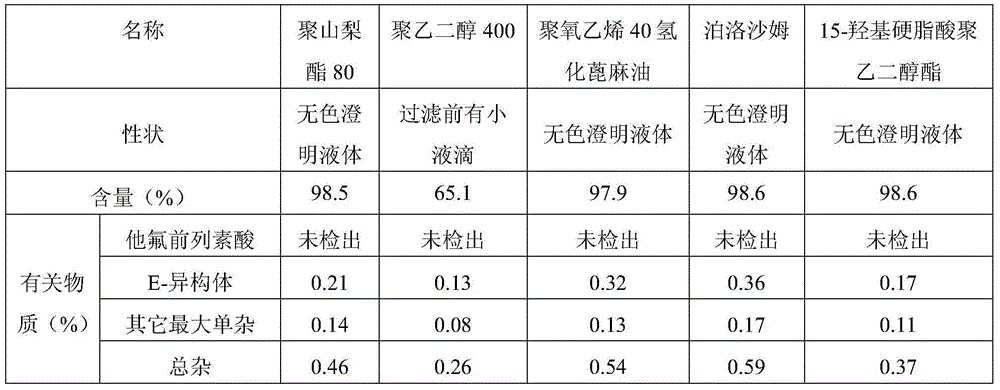
[0042] Embodiment 1: Preparation of multi-dose tafluprost ophthalmic preparation containing bacteriostatic agent
[0043] prescription:
[0044] Element
[0045] Preparation method: Take benzalkonium chloride and add 9 times the amount of water, stir to dissolve completely and mix evenly to obtain an aqueous solution of about 10% benzalkonium chloride; take 15-hydroxypolyethylene glycol stearate and add 2.5ml for injection Stir with water, dissolve, add tafluprost, and stir until the tafluprost is completely dissolved to obtain a premixed solution containing tafluprost. Add the premixed solution of tafluprost to 4000ml of water for injection, then add 10% benzalkonium chloride aqueous solution, edetate disodium, sodium dihydrogen phosphate, glycerin, stir to dissolve completely and mix evenly, use 0.1 mol / L sodium hydroxide solution or / and 0.1mol / L hydrochloric acid solution to adjust the pH value of the liquid to about 5.5-6.0, add water for injection to the full ...
Embodiment 2
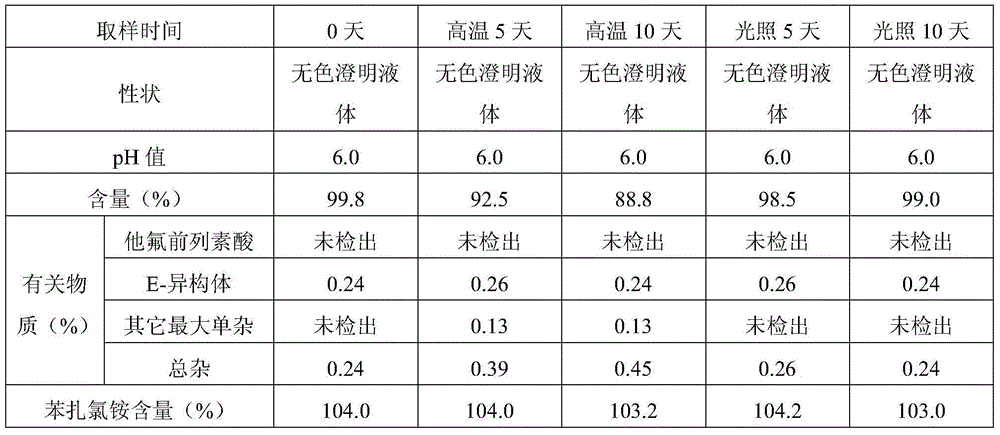
[0046] Embodiment 2 Preparation of multi-dose tafluprost ophthalmic preparation containing bacteriostatic agent
[0047] prescription:
[0048] Element
[0049] Preparation method: take benzalkonium chloride and add 9 times the amount of water, stir to dissolve completely and mix evenly to obtain an aqueous solution of about 10% benzalkonium chloride; take 15-hydroxypolyethylene glycol stearate and add 50ml water for injection dissolve, add tafluprost, and stir until the tafluprost is completely dissolved to obtain a premixed solution containing tafluprost. Add the premixed solution of tafluprost into 8000ml of water for injection, then add 10% aqueous solution of benzalkonium chloride, sodium calcium edetate, sodium dihydrogen phosphate, glycerin, stir to completely dissolve and mix, and use 0.1 mol / L sodium hydroxide solution or / and 0.1mol / L hydrochloric acid solution to adjust the pH value of the liquid to about 5.8-6.6, add water for injection to the full amoun...
Embodiment 3
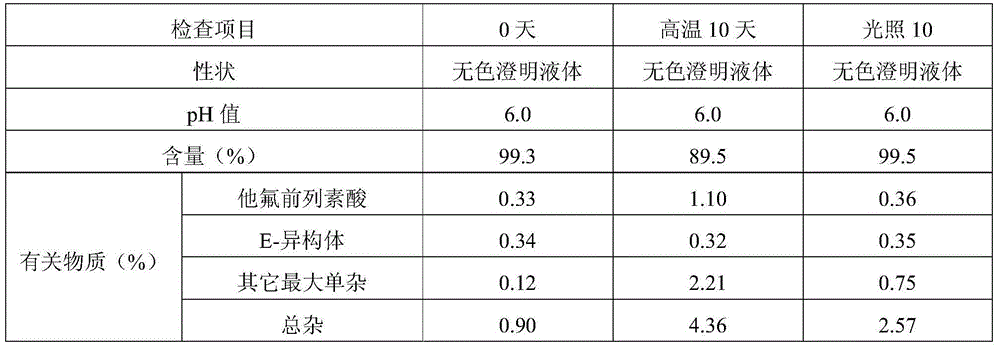
[0050] Embodiment 3 Preparation of multi-dose tafluprost ophthalmic preparation containing bacteriostatic agent
[0051] prescription:
[0052] Element
[0053] Preparation method: take benzalkonium chloride and add 9 times the amount of water, stir to dissolve completely and mix evenly to obtain an aqueous solution of about 10% benzalkonium chloride; take 15-hydroxypolyethylene glycol stearate and add 200ml water for injection dissolve, add tafluprost, and stir until the tafluprost is completely dissolved to obtain a premixed solution containing tafluprost. Add 10% benzalkonium chloride aqueous solution, edetate disodium, sodium dihydrogen phosphate, and sodium chloride to 7000ml of water for injection, stir to dissolve completely and mix well, and use 0.1mol / L sodium hydroxide solution or / and 0.1mol / L hydrochloric acid solution to adjust the pH value of the liquid to about 6.0-6.5, add the premixed solution of tafluprost, add water for injection to the full amo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com