Method for synthesizing tafluprost
A technology for tafluprost and compounds, which is applied in the field of synthesizing tafluprost, can solve the problems of low production efficiency, high cost, low purity, etc., and achieve the effect of precise control of reaction conditions and improvement of atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
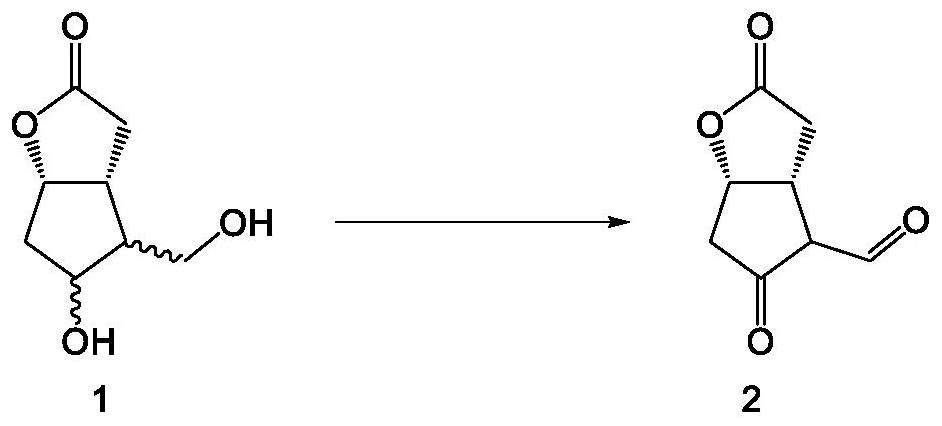
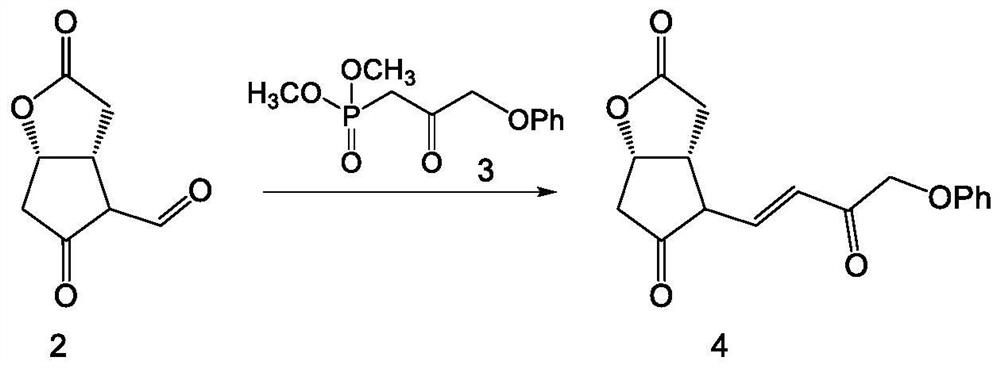
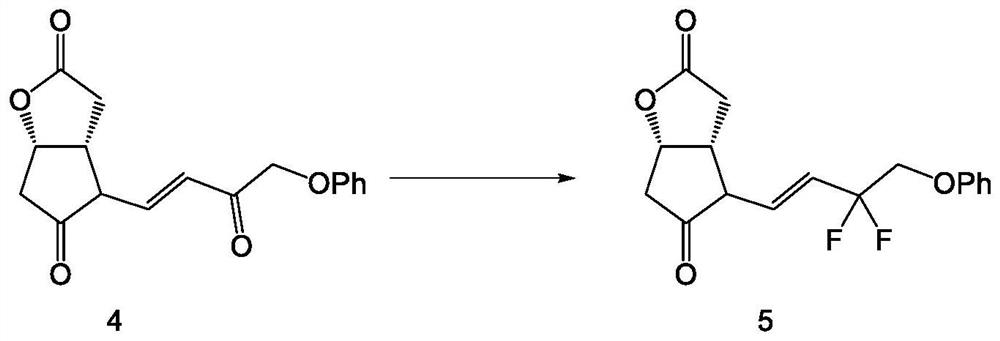
[0038] Such as Figure 1-6 As shown, the embodiment of the present invention provides a method for synthesizing tafluprost, comprising the following specific preparation steps:
[0039] S1. Preliminary reaction, the preliminary medicament is obtained through the oxidation reaction of the original compound, specifically, 9.0g, 1.2eq of NaOCl oxidant, 1.5g, 0.1eq of TEMPO catalyst and 11.7g of NaBr, 1.0eq are added to DCM100mL, stirred for 15 minutes, Cool down to -10-0°C, add 17.2g of the compound of formula 1 in DCM100mL solution dropwise to the above reaction mixture, keep stirring at -10-0°C for 3h, add DCM300mL to dilute, add 10% sodium thiosulfate aqueous solution Perform quenching, keep stirring at 0-10°C for 1 hour, separate the liquids, combine the organic phases, and dry the organic phases with anhydrous sodium sulfate to obtain a preliminary medicament;
[0040] S2. Secondary reaction, the secondary reaction compound is obtained through the Horner-Wadsworth-Emmons re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com