Preparation method of pramipexole hydrochloride
A technology of pramipexole hydrochloride and amino, which is applied in the field of pharmaceutical preparations, can solve the problems of difficult industrial production of pramipexole hydrochloride, low yield, high environmental pollution, etc., achieve broad prospects and industrialization should be valuable, and increase the yield , The effect of cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
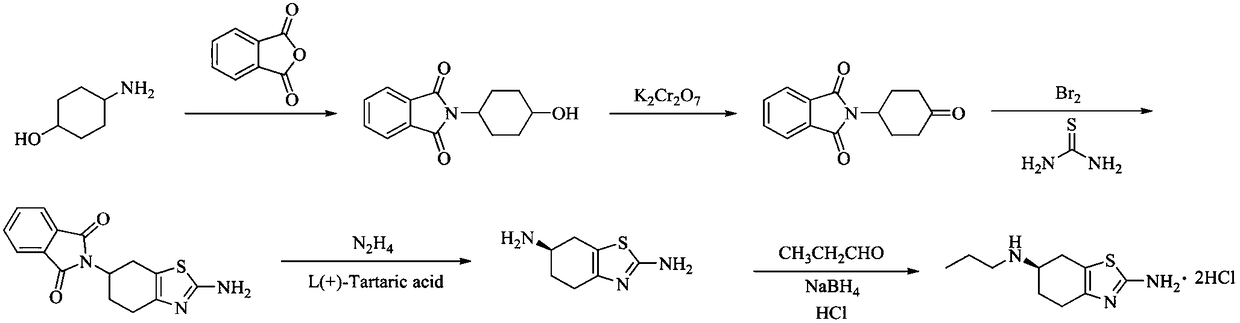
[0035] The preparation method of a kind of pramipexole hydrochloride proposed by the present invention comprises the following steps: first, p-aminocyclohexanol is condensed and protected with propionyl chloride to obtain p-propionyl cyclohexanol, and then obtained p-propionyl cyclohexanone through catalytic oxidation , followed by oxidative bromination and Hantzsch condensation to obtain 2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole, and then reduced by diisobutylaluminum hydride to obtain 2-amino-6- Propylamino-4,5,6,7-tetrahydrobenzothiazole, and then use L-(+)-tartaric acid as a resolving agent to carry out a resolution reaction, and finally form a salt to obtain pramipexole hydrochloride.
Embodiment 2
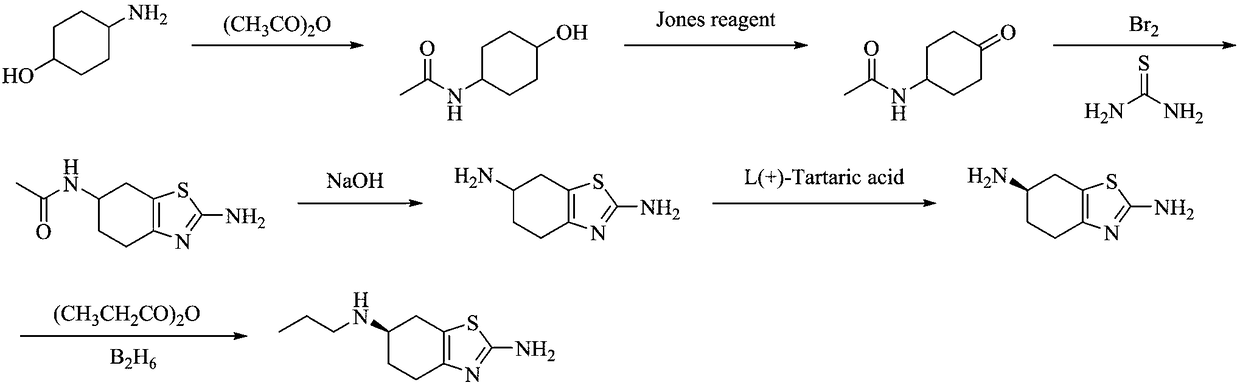
[0037] The preparation method of a kind of pramipexole hydrochloride proposed by the present invention comprises the following steps: firstly protect the amino group of p-aminocyclohexanol through propionyl chloride condensation to obtain p-propionyl cyclohexanol, wherein, the mixture of propionyl chloride and p-aminocyclohexanol The molar ratio between them is 1:1, and the reaction temperature is -30°C; then, p-propionylcyclohexanone is obtained through catalytic oxidation, wherein, in the catalytic oxidation process, TCCA is selected as the oxidant, TEMPO and NaBr are selected as the catalyst, and dichloro Methane is the solvent, and the molar ratio between propionyl cyclohexanol, TCCA, TEMPO, and NaBr is 2:1:0.02:0.2, and the reaction temperature is -10°C; then it is obtained by one-pot method of oxidative bromination and Hantzsch condensation 2-Amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole, wherein, in the oxidative bromination process, the oxidative bromination re...
Embodiment 3
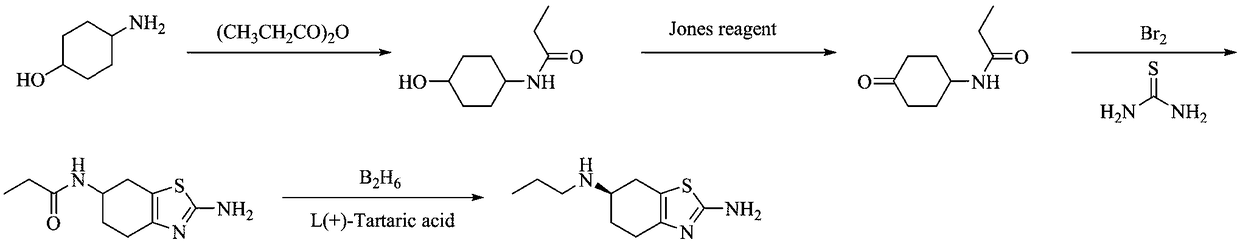
[0039] The preparation method of a kind of pramipexole hydrochloride proposed by the present invention comprises the following steps: firstly protect the amino group of p-aminocyclohexanol through propionyl chloride condensation to obtain p-propionyl cyclohexanol, wherein, the mixture of propionyl chloride and p-aminocyclohexanol The molar ratio between them is 1.5:1, and the reaction temperature is 30°C; then, p-propionylcyclohexanone is obtained through catalytic oxidation, wherein, in the catalytic oxidation process, TCCA is selected as the oxidant, TEMPO and NaBr are selected as the catalyst, and dichloromethane as a solvent, and the molar ratio between propionyl cyclohexanol, TCCA, TEMPO, and NaBr is 1:0.5:0.01:0.1, and the reaction temperature is 40°C; then 2- Amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole, wherein, in the oxidative bromination process, the oxidative bromination reagent is H 2 o 2 / HBr; and then reduced by diisobutyl aluminum hydride to obtain 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com