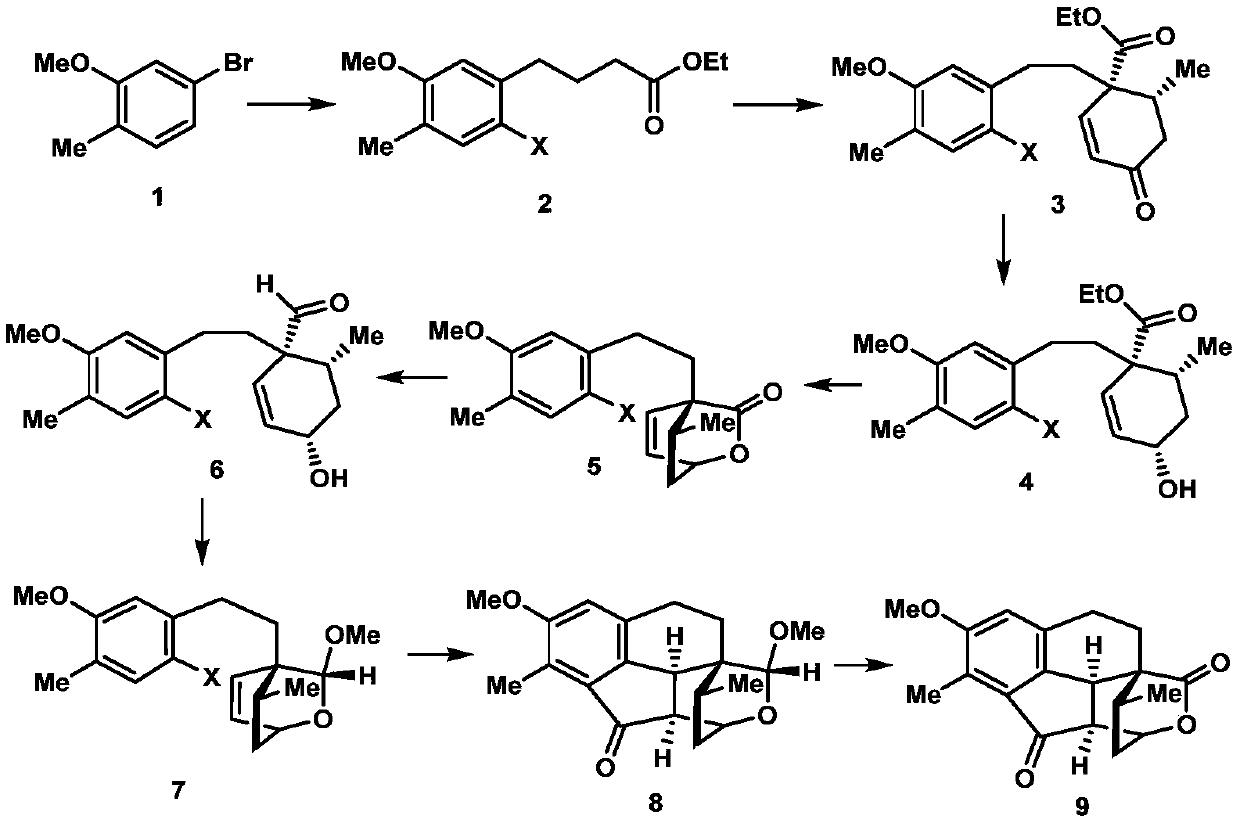
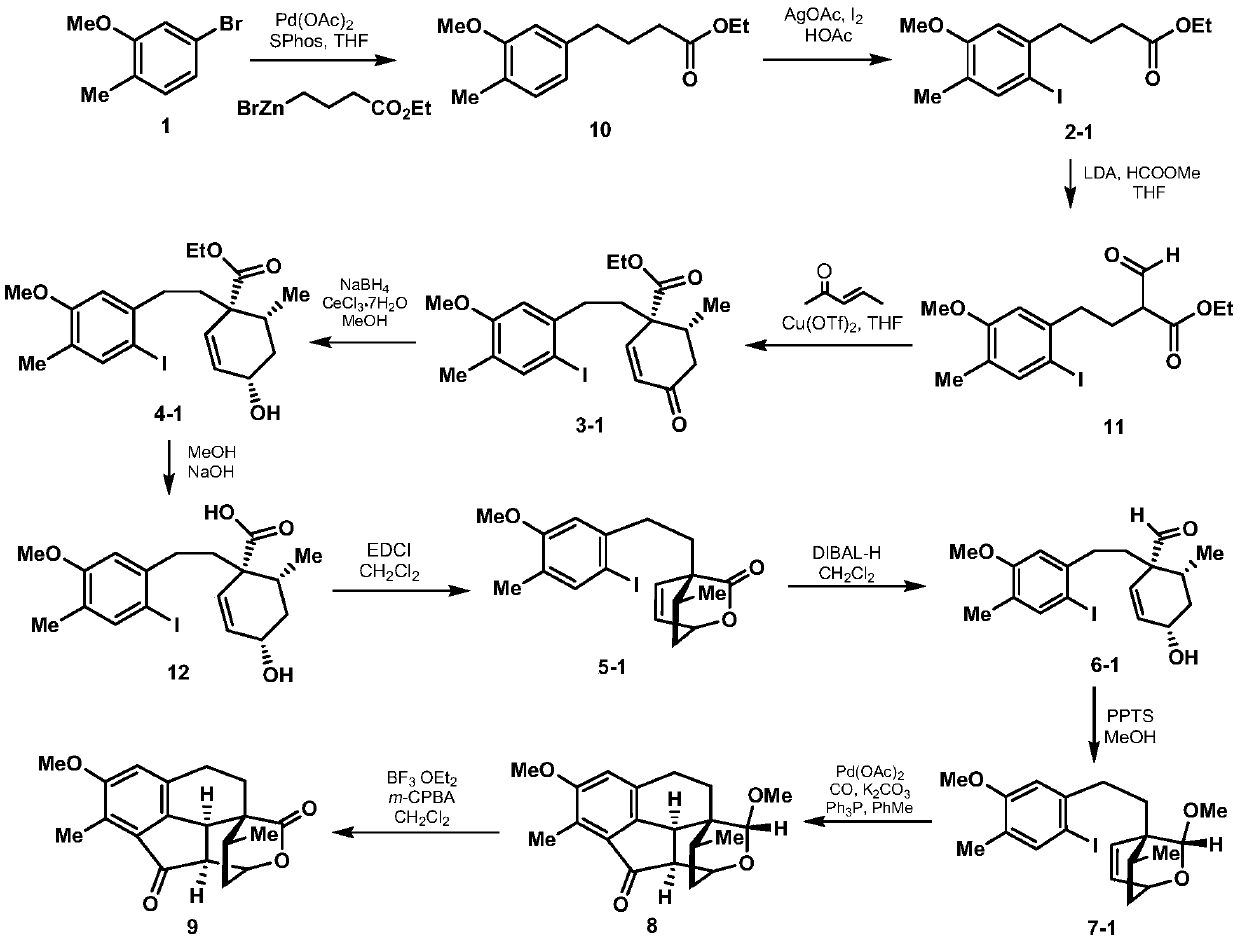
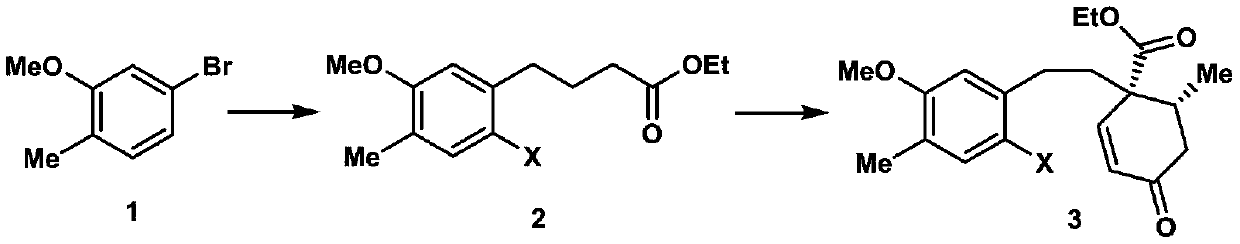
A kind of synthetic method of crude torene terpenoid skeleton compound
A technology of a skeleton compound and a synthetic method, applied in the field of synthesis of natural products, can solve problems such as reports on the chemical synthesis method of crude torreya-like diterpenoid natural products, and achieve the effects of simple and efficient synthetic route, easy operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018]
[0019] 1. Add 24.1g (120mmol) of 5-bromo-2-methylanisole shown in formula 1, 539mg (2.4mmol) of palladium acetate, 1.1g (4.8mmol) of S-Phos in a 500mL dry round bottom flask , then add 50mL of degassed tetrahydrofuran, stir at room temperature for 5 minutes under the protection of nitrogen, then add 300mL of 0.6mol / L tetrahydrofuran solution of ethyl 4-bromobutyrate zinc reagent, stir at 50°C for 5 hours, and then use ethyl acetate and saturated chlorine The ammonium chloride solution was extracted, the organic phase was collected, dried over sodium sulfate, spin-dried, and passed through the column with petroleum ether and ethyl acetate to obtain 27.8 g of the compound shown in formula 10, with a yield of 98%. The structural characterization data are as follows: 1 HNMR (600MHz, deuterated chloroform) δ7.07(d, J=7.5Hz, 1H), 6.71(d, J=7.5Hz, 1H), 6.69(s, 1H), 4.16(q, J=7.6, 7.2 Hz, 2H), 3.85(s, 3H), 2.66(t, J=7.7Hz, 2H), 2.36(t, J=7.5Hz, 2H), 2.23(s, 3H), 1.99(p, J=...
Embodiment 2
[0031] In step 2 of Example 1, the 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride used is replaced with equimolar dicyclohexylcarbodiimide, other steps of this step Same as Example 1, the lactone compound represented by formula 5-1 was obtained, and the total yield of the two steps was 57%. Other steps are identical with embodiment 1.
Embodiment 3
[0033] In step 3 of Example 1, the pyridinium p-toluenesulfonic acid used is replaced with equimolar p-toluenesulfonic acid, and the other steps of this step are the same as in Example 1 to obtain the acetal compound shown in Formula 7-1, with a yield of 58%. Other steps are identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com