Patents
Literature
42 results about "Bromoanisole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
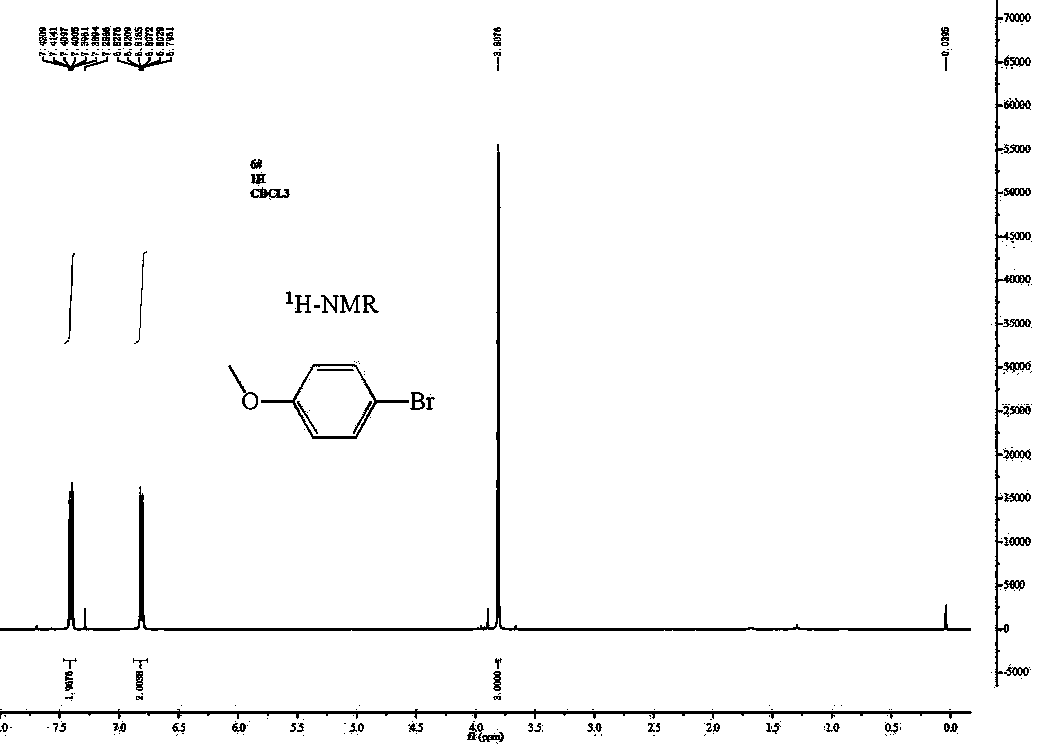
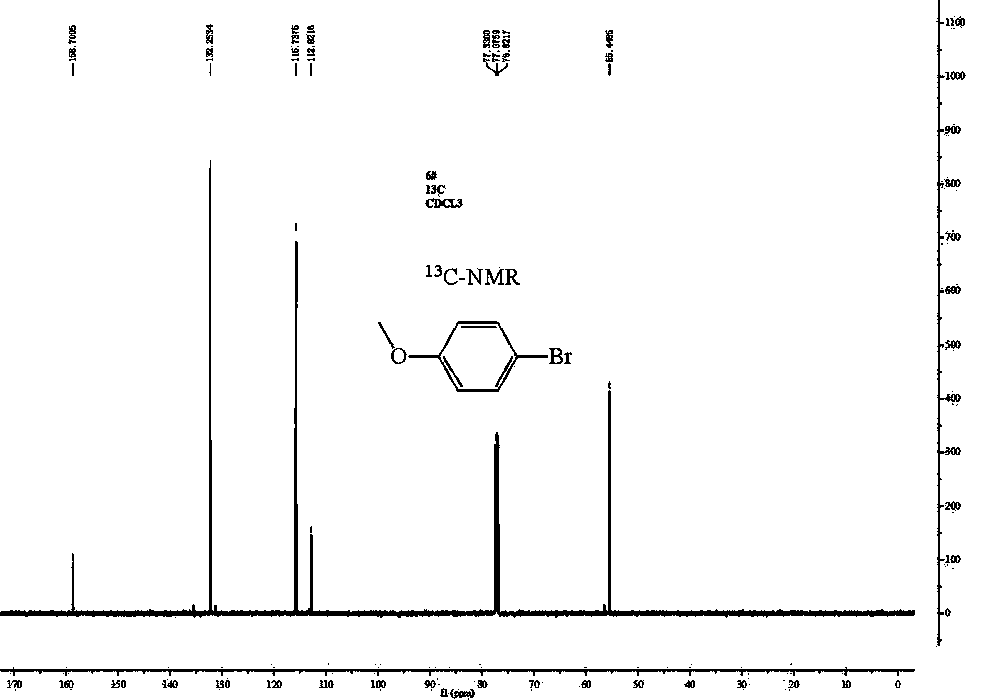
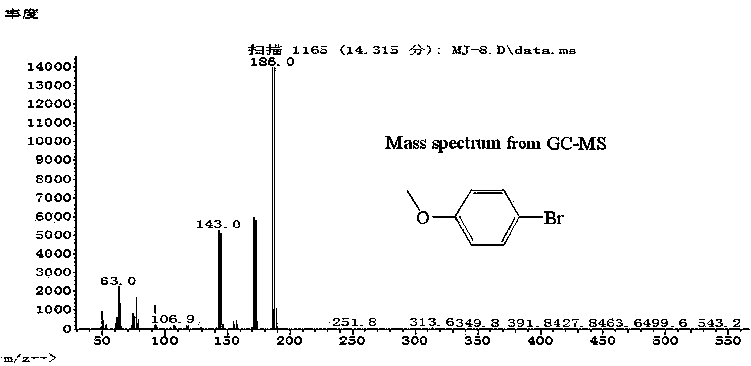
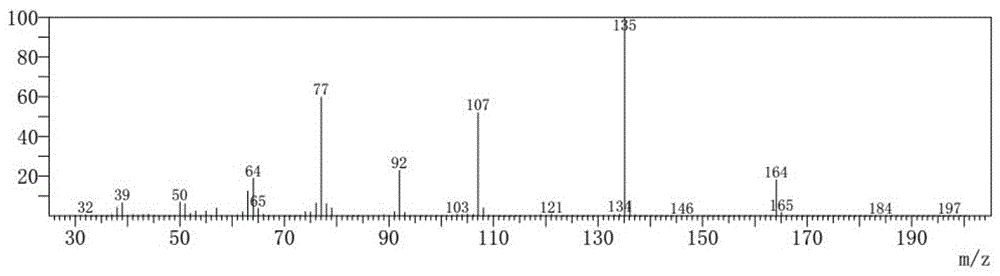
4-Bromoanisole, also known as para-bromoanisole or 1-bromo-4-methoxybenzene, is a clear liquid with a pleasant smell similar to that of anise seed. 4-Bromoanisole is an optional compound sometimes used in RNA extraction which serves to further eliminate DNA contamination. It will interact with genomic DNA (gDNA) and through a separation phase, it will be located in the organic layer instead of the aqueous layer (upper layer) containing the RNA extract.
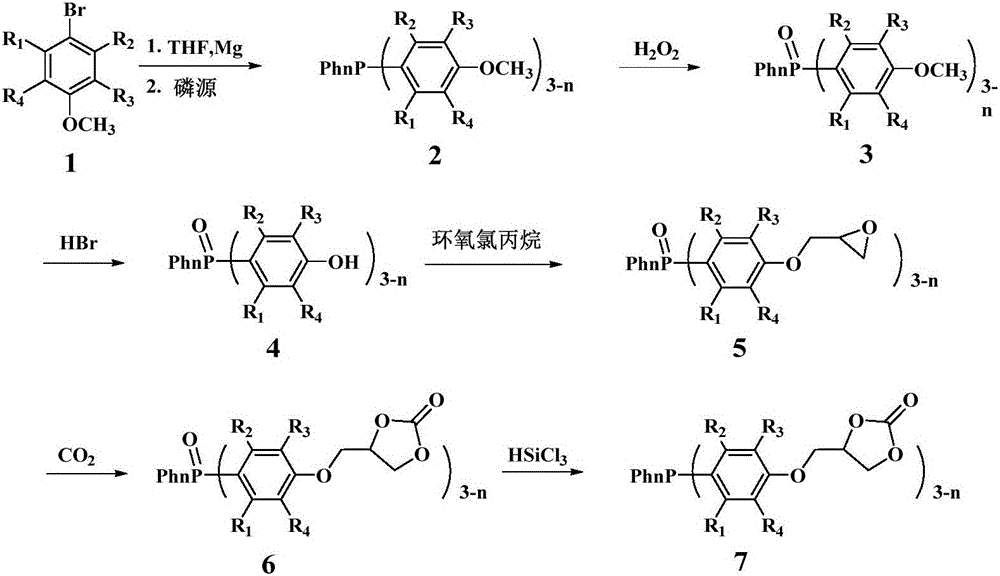
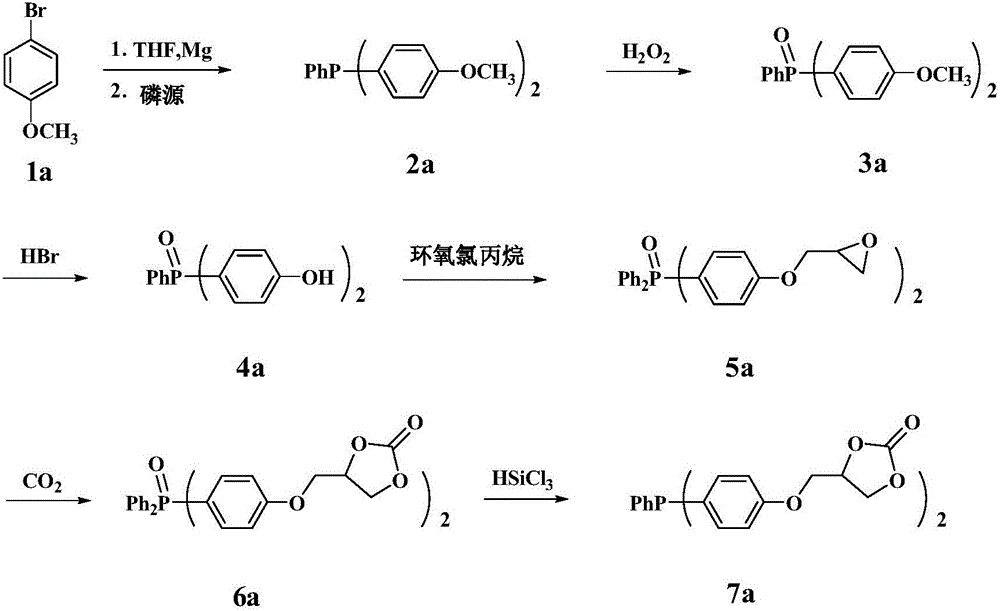
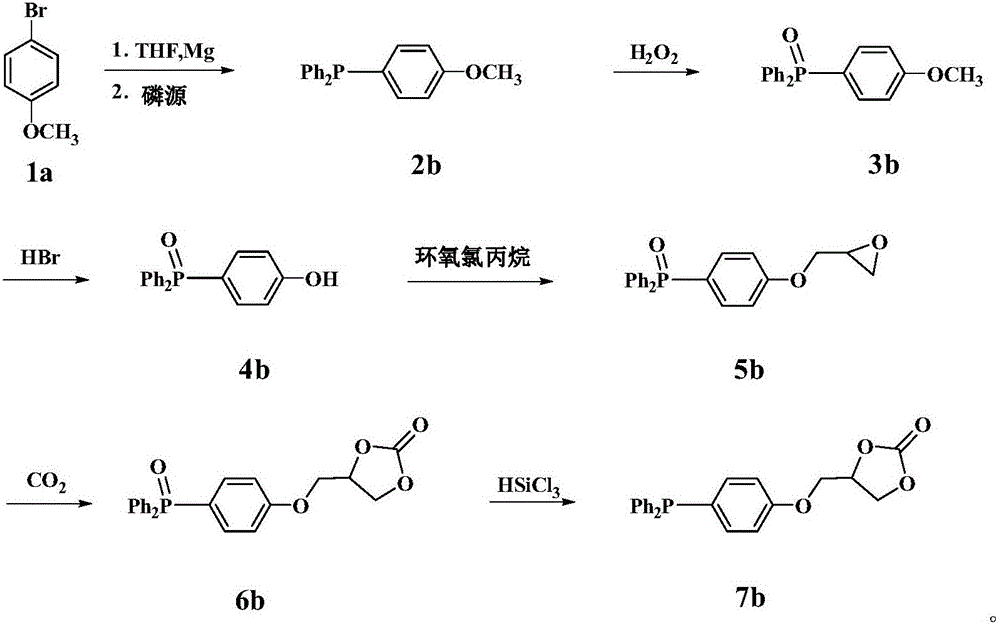
Derivative of dibenzofuran and preparation method and application thereof
ActiveCN105153085AImprove matchImprove thermal stabilityOrganic chemistrySolid-state devicesVitrificationPerovskite solar cell
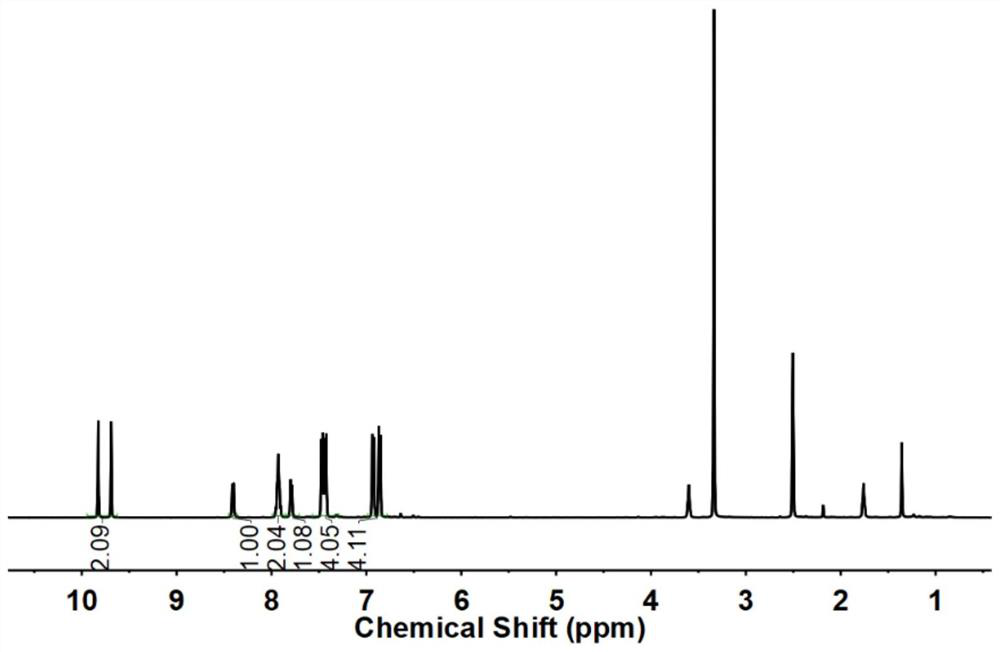
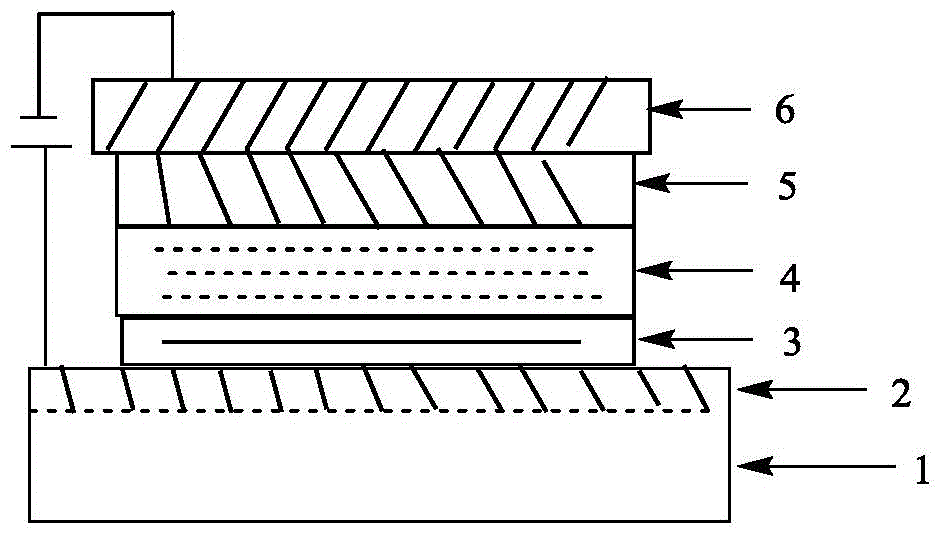
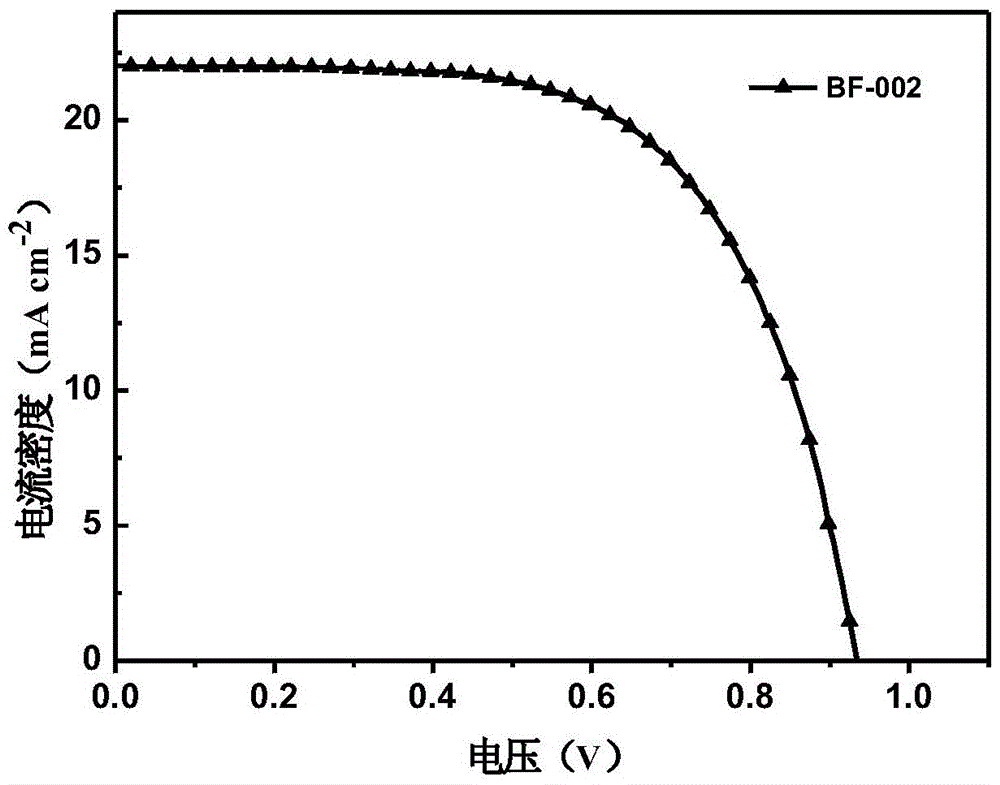
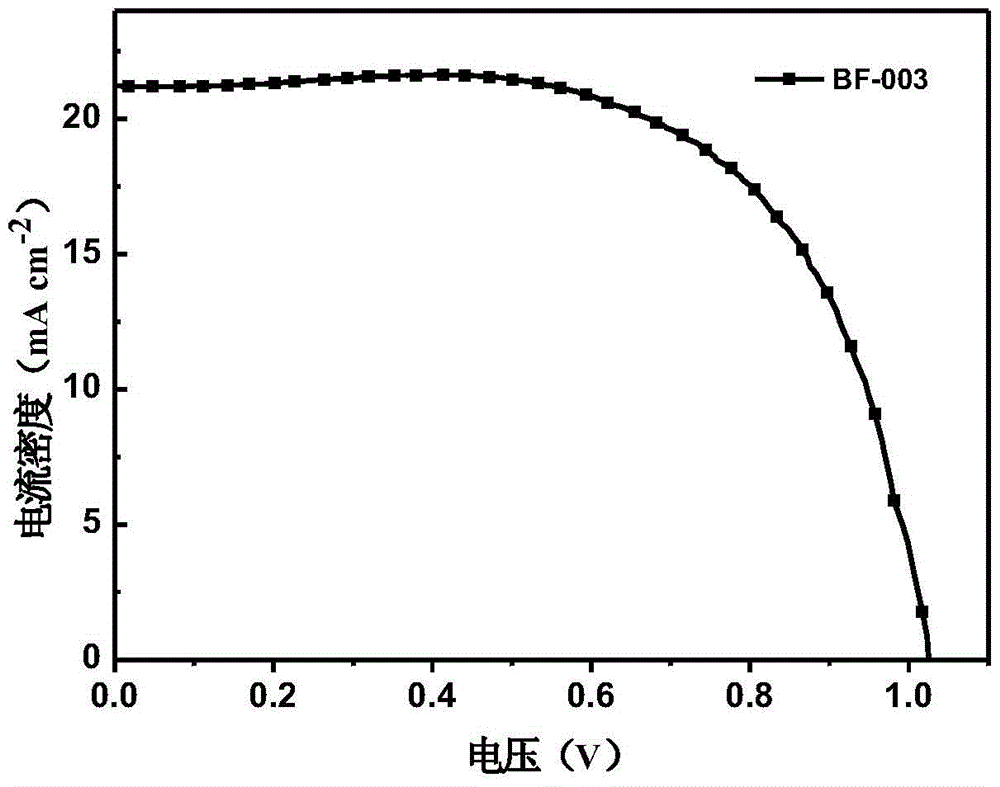
The invention relates to a derivative of dibenzofuran and a preparation method and application thereof. The preparation method of the derivative of the dibenzofuran comprises the steps that carbon-nitrogen coupling is performed on p-bromoanisole and p-methoxyaniline to obtain a first precursor; a two-step reaction is performed on the first precursor and 4,4'-dibromodiphenylamine protected by butyloxycarbonyl to obtain a second precursor, and a two-step reaction is performed on the first precursor and 3,6-dibromocarbazole protected by butyloxycarbonyl to obtain a third precursor; carbon-nitrogen coupling is performed on all the precursors and 2,8-dibromodibenzofuran under the action of a palladium catalyst to obtain the derivative of the dibenzofuran. The prepared derivative of the dibenzofuran is easy to synthesize, low in cost, high in glass transition temperature and good in heat stability, is a hole-transport material with the good properties and has the good effect when the derivative is applied to perovskite solar cells.
Owner:VALIANT CO LTD
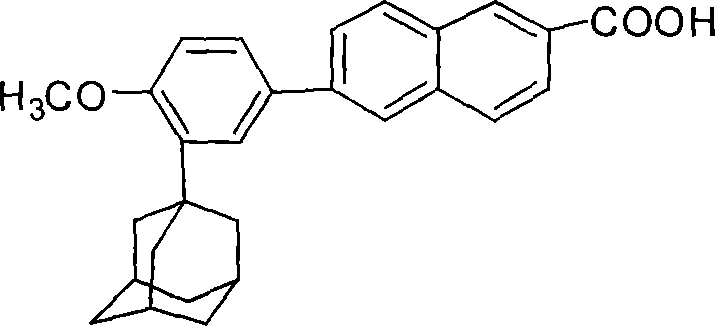
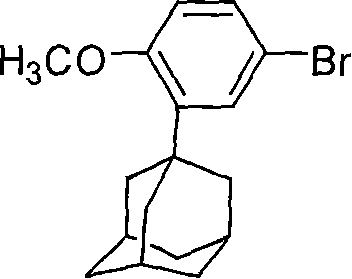
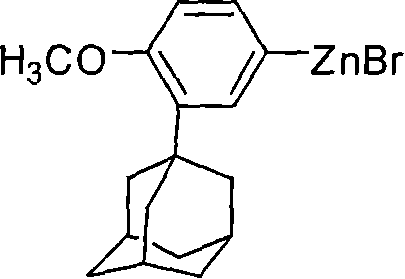
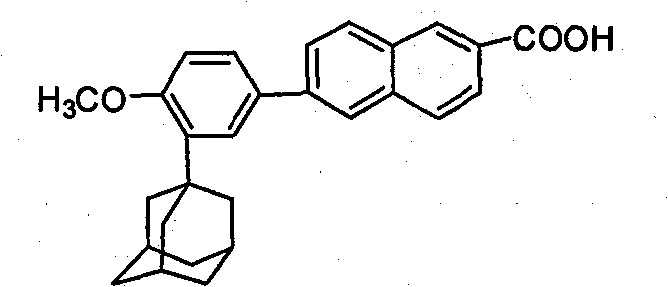
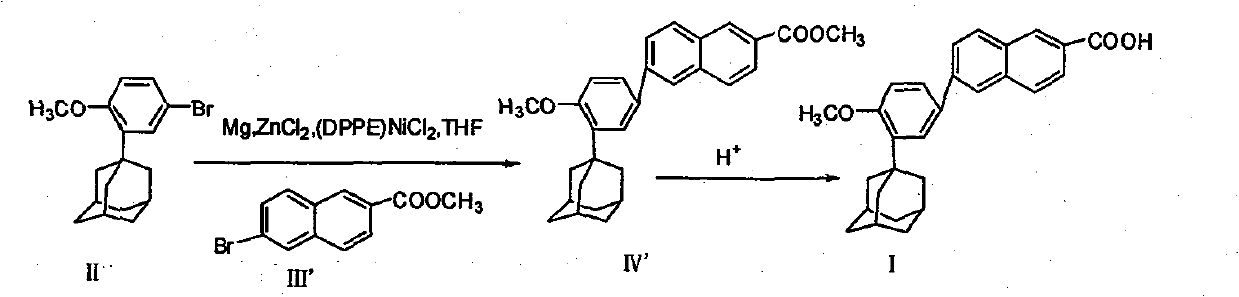
Process for preparing 6-[3-(1-adamantyl)-4-methoxy phenyl]-2-methyl naphthoate
InactiveCN1827582AQuality improvementHigh yieldOrganic compound preparationCarboxylic acid esters preparationIodideGrignard reagent
The invention relates to a method for preparation of methyl 6-[3-(1-adamantyl)-4-methoxylphenyl]-2- naphthoate. It consisits of synthesizing 2-(1-adamantyl)-4-bromophenol by coupling reaction between bromophenol and adamantine alcohol, synthesizing 2-(1-adamantyl)-4-bromoanisole by methylating reaction between the obtained 2-(1-adamantyl)-4-bromophenol and the methyliodide, then gaining the Grignard reagent by 2-(1-adamantyl)-4-bromoanisole and magnesium initiated by one or more than one of iodine, iodide and bromide in the solvent of ethers or non-polar solvent, and finally synthesizing the said methyl 6-[3-(1-adamantyl)-4-methoxylphenyl]-2-naphthoate by reaction between methyl 6- bromine-2-naphthoate and the said Grignard reagent. The invention is characterized in that its side reaction is decreased, its product quality is improved, its stocks are all normal chemical products of localization of manufactures, its operation is simple, and the three wastes are reduced, the cost is reduced, so it is suitable for industrial production of large scale.
Owner:JIANGSU ZHONGDAN GROUP +2
Process for preparing 2-[(dimethylamino)-methyl]-1-(3-methoxyphenyl)cyclohexanol
InactiveUS7030276B2High stereoselectivityHigh yieldOrganic compound preparationAmino compound preparationLithiumCyclohexanone
A process for preparing 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol with high stereoselectivity and high yield by reacting 2-[(dimethylamino)methyl]-cyclohexanone in a Grignard reaction with a Grignard compound of 3-bromoanisole in a suitable solvent and in the presence of an inorganic lithium salt and an α,ω-dialkoxyalkane.
Owner:GRUNENTHAL GMBH
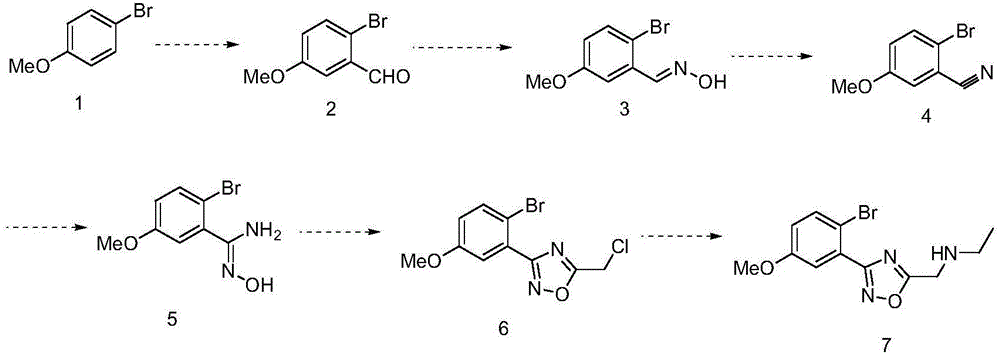
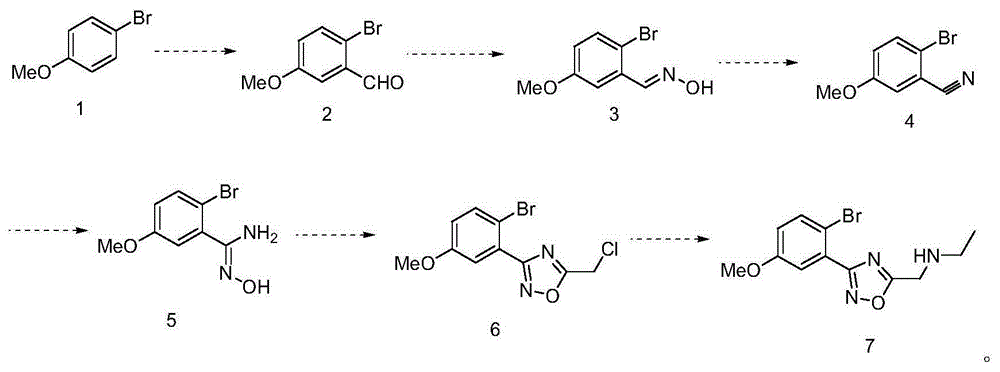
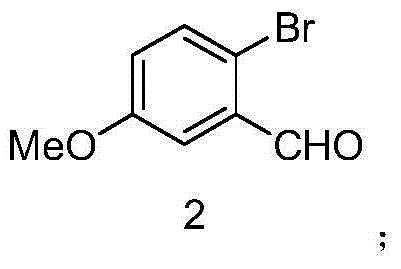
Preparation method of benzene substituent oxadiazole compound
The invention discloses a preparation method of a benzene substituent oxadiazole compound N-((3-(2-bromine-5-methoxy phenyl)-1, 2, 4-oxadiazole-5-radical) methyl) ethylamine. 4-bromoanisole serves as a starting material and is subjected to aldehydes, oximate, elimination, addition, cyclization and substitution reaction to obtain a target product 7. The product serving as a template micro-molecule for synthesizing diversified compound libraries.
Owner:湖南华腾制药有限公司
Synthesis method for 3-methoxypropiophenone
InactiveCN106518635ALow costEasy to operateOrganic compound preparationCarbonyl compound preparation by condensationGrignard reagentSynthesis methods
The invention discloses a synthesis method for 3-methoxypropiophenone. The synthesis method comprises the steps of enabling magnesium and m-bromoanisole to produce a Grignard reagent in a tetrahydrofuran (THF) solution under the catalytic action of aluminum chloride, and then, subjecting the Grignard reagent to a reaction with propionitrile, thereby producing the 3-methoxypropiophenone. According to the synthesis method, the operation is simple, the process is advanced, the solvent can be recycled, and thus, the industrial production is facilitated. According to the 3-methoxypropiophenone prepared by the synthesis method, the yield reaches 88.6%, and the liquid-phase purity reaches up to 99.44% or more.
Owner:CHINA THREE GORGES UNIV
Process for preparing 2-[(dimethylamino)-methyl]-1-(3-methoxyphenyl)cyclohexanol
InactiveUS20050215821A1High stereoselectivityHigh yieldSilicon organic compoundsOrganic compound preparationCyclohexanoneLithium
A process for preparing 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol with high stereoselectivity and high yield by reacting 2-[(dimethylamino)methyl]-cyclohexanone in a Grignard reaction with a Grignard compound of 3-bromoanisole in a suitable solvent and in the presence of an inorganic lithium salt and an α,ω-dialkoxyalkane.
Owner:GRUNENTHAL GMBH
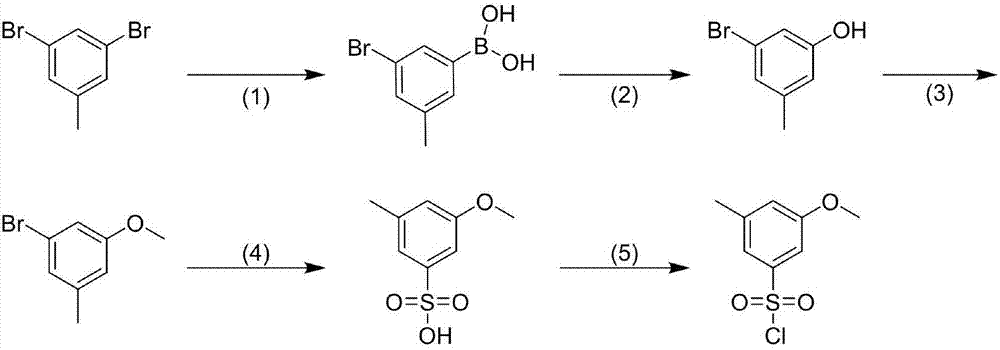
Preparation process for 3-methyl-5-methoxybenzenesulfonyl chloride
InactiveCN107501132AEasy access to raw materialsEasy post-processingSulfonic acid preparationChlorideSubstitution reaction
The invention discloses a preparation process for 3-methyl-5-methoxybenzenesulfonyl chloride. The process comprises the following steps: 3,5-dibromotoluene is used as an initial raw material, the 3,5-dibromotoluene is subjected to a boronic acidification reaction, thus 3-methyl-5-bromophenylboronic acid is obtained, the 3-methyl-5-bromophenylboronic acid is subjected to an oxidation reaction, thus 3-methyl-5-bromophenol is obtained, the 3-methyl-5-bromophenol is subjected to a substitution reaction, thus 3-methyl-5-bromoanisoles is obtained, the 3-methyl-5-bromoanisoles is subjected to sulfonation, thus 3-methyl-5-methoxybenzenesulfonate is obtained, the 3-methyl-5-methoxybenzenesulfonate is subjected to a substitution reaction, and therefore the objective compound 3-methyl-5-methoxybenzenesulfonyl chloride is obtained. According to the above route, the raw materials are easy to obtain, post-treatment is simple, convenient and easy to implement, a yield is relatively high, and the process has relatively-good application value.
Owner:GUIZHOU UNIV
Preparation method of bisphenol monomer containing phthalazinone structure
The invention discloses a preparation method of a bisphenol monomer containing a phthalazinone structure, and belongs to the technical field of novel materials. The bisphenol monomer containing the phthalazinone structure is synthesized by a two-step reaction. The method comprises the following steps: a bisphenol-like monomer containing the phthalazinone structure and p-bromoanisole are used as raw materials, 1,10-phenanthroline hydrate is adopted as a N-N bidentate ligand, CuI is used as a catalyst, a C-O, C-N Ullmann coupling reaction is performed to obtain intermediate product DMPPZ, and Lewis acid reduction is performed to obtain the target monomer DHPPZ. The synthetic method provided by the invention has the advantages of a short synthetic period and mild conditions; and the purity ofthe target monomer tested by liquid chromatography-mass spectrometry is 98%, and the yield is 85%-90%.
Owner:DALIAN UNIV OF TECH
Synthesis method of carbonate modified fluoride-free organic phosphine ligand
InactiveCN106543224AImprove solubilityAchieve recyclingGroup 5/15 element organic compoundsBulk chemical productionSolubilityOrganometallic catalysis
The invention relates to the field of organic synthesis and provides a synthesis method of a carbonate modified fluoride-free organic phosphine ligand. The synthesis method comprises the following steps: preparing a Grignard reagent from the raw material p-bromoanisole or derivative thereof; enabling the Grignard reagent to react with a phosphorus source to generate a skeleton structure molecule with triphenylphosphine; and performing oxidation, demethylation, nucleophilic substitution and reduction to obtain a target molecular structure. The synthesis method has the following effects and benefits: a novel carbon dioxide-philic organic phosphine ligand is synthesized, the solubility of the phosphine ligand in supercritical CO2 is increased, and application of a fluoride-free phosphine ligand organic metal catalyst in supercritical CO2 is realized. Moreover, the organic carbonate compound is insoluble in a weakly (non-) polar organic solvent (such as alkane compounds), the property enables a phase splitting function of the carbonate modified fluoride-free organic phosphine ligand in the reaction system, and the recycling and reusing of the catalyst are realized.
Owner:DALIAN UNIV OF TECH
Production technology for synthesizing m-bromoanisole
InactiveCN104045525AEasy to useEasy to prepareEther preparation by ester reactionsChemical industryDistillation
A production technology for synthesizing m-bromoanisole relates to the technical filed of the chemical industry. The technology comprises the following steps: weighing a sodium hydroxide solution and an m-bromophenol solution, adding the sodium hydroxide solution and the m-bromophenol solution into a reaction container, stirring a mixture for dissolving, introducing steam for heating, adding dimethyl sulfate in a dropwise manner, adding the obtained cooled product to an oil-water separator, allowing the product to stand for separating in order to obtain an oil layer and a water layer, extracting the water layer by ether, drying the obtained ether layer by anhydrous calcium chloride, adding the dried ether layer to a distillation kettle, distilling, mixing residues obtained after the distillation with the oil layer for later use, adding the obtained residue and oil layer mixture to a rectifying tower, carrying out reduced pressure distillation, and collecting the obtained fraction which is the m-bromoanisole finished product. The technology has the advantages of convenient and simple preparation, environmental protection, no pollution, easily available materials, less equipment investment, high purity and convenient operation, and the prepared m-bromoanisole has the advantages of good use effect, safety and reliability.
Owner:ANHUI HUARUN PAINTS
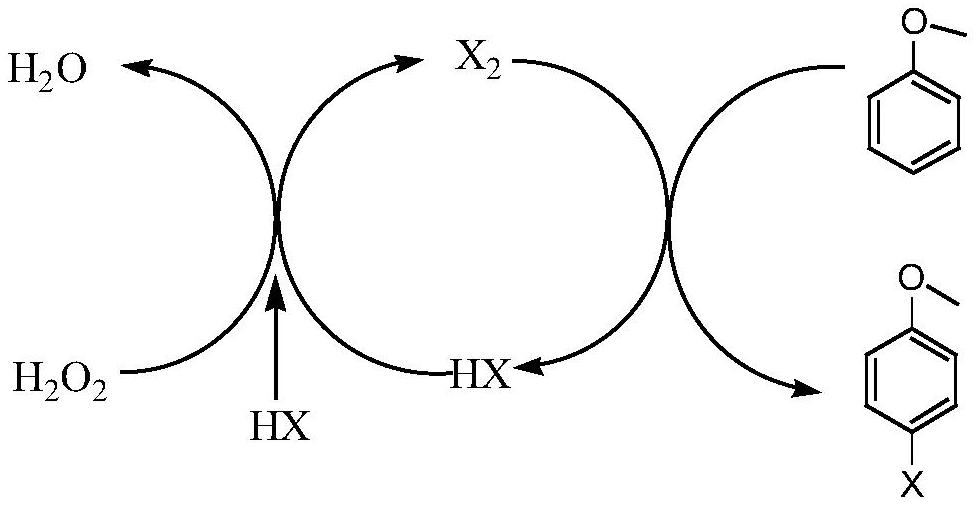
Method for preparing p-bromoanisole by oxidative bromination method
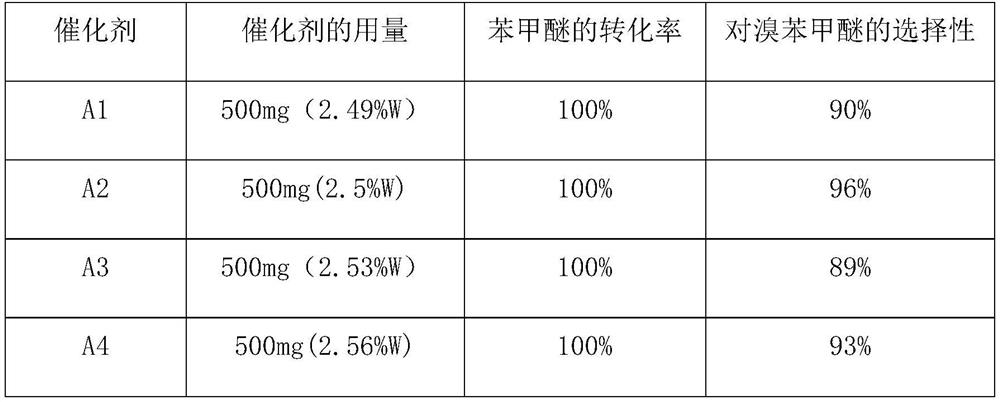
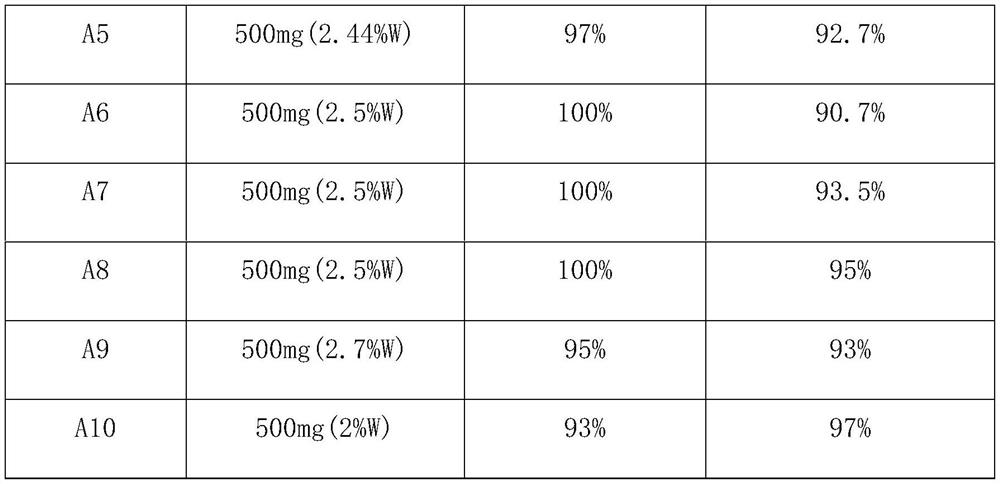
ActiveCN114751809ASimple preparation processHigh activityPhysical/chemical process catalystsOrganic chemistryPtru catalystCombinatorial chemistry
The invention provides a method for preparing bromoanisole by an oxidative bromination method, which comprises the following steps of: oxidizing hydrobromic acid by anisole under the action of a catalyst and hydrogen peroxide, and brominating the anisole to obtain p-bromoanisole. The catalyst is a nitrogen-containing carbon material immobilized phosphotungstic acid catalyst, is simple to prepare, has relatively high activity and selectivity, is good in stability, can be repeatedly used for multiple times, can be used for catalyzing highly selective oxidative bromination of anisole to prepare p-bromoanisole, and has a very good industrial application prospect.
Owner:NANJING TECH UNIV
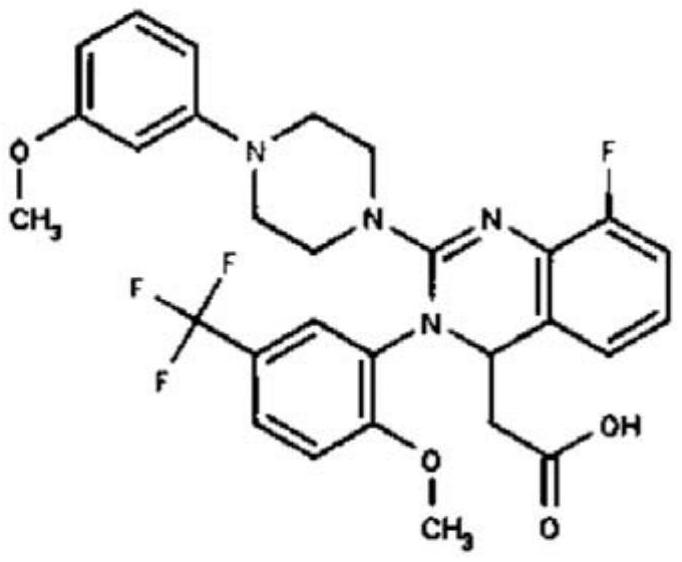
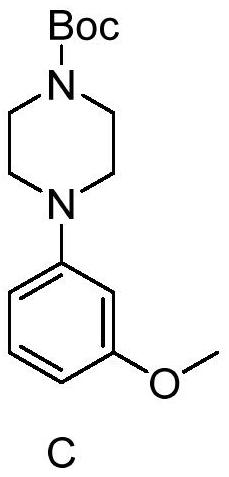
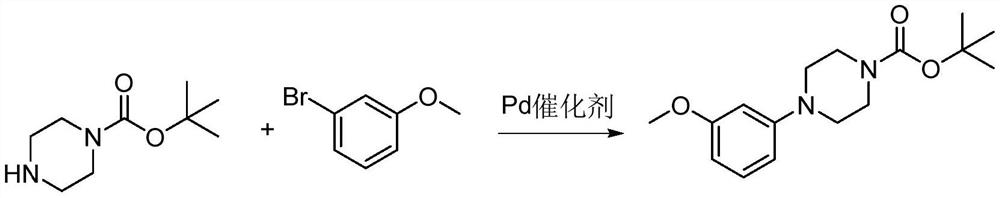
Method for synthesizing letemovir intermediate
The invention relates to a method for synthesizing a letemovir intermediate, belongs to the field of organic chemical synthesis, and provides a novel method for preparing the letemovir intermediate in order to mainly solve the problems of large potential safety hazard, high production cost, unsuitability for industrial large-scale production, low conversion rate and the like. Comprising the following steps: 1) adding 4-fluoroanisole or 4-bromoanisole, 1-t-butyloxycarbonyl piperazine and an organic solvent into a reaction flask; 2) adding strong base; 3) reacting for 20 hours at 95-150 DEG C under the protection of nitrogen; and 4) observing the reaction degree through HPLC. Compared with the prior art (palladium catalytic coupling), the technical scheme provided by the invention is better in safety and lower in cost; according to the scheme provided by the invention, no copper salt is used for catalysis in the preparation process, the reaction effect is better only under a strong alkali condition, and the reaction conversion rate is increased to 62% at present from 0-26% when a copper catalyst is used.
Owner:RAFFLES PHAMRMATECH CO LTD
Process for the preparation of tapentadol
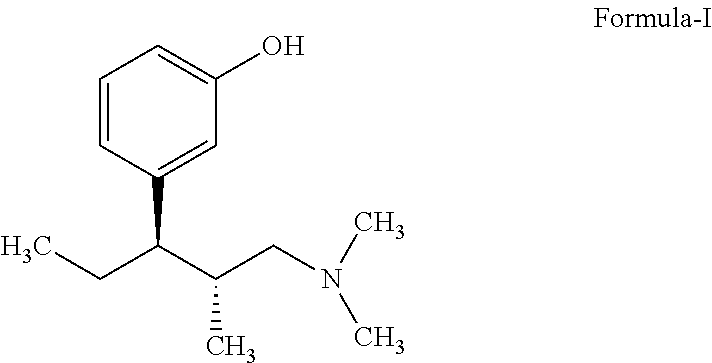
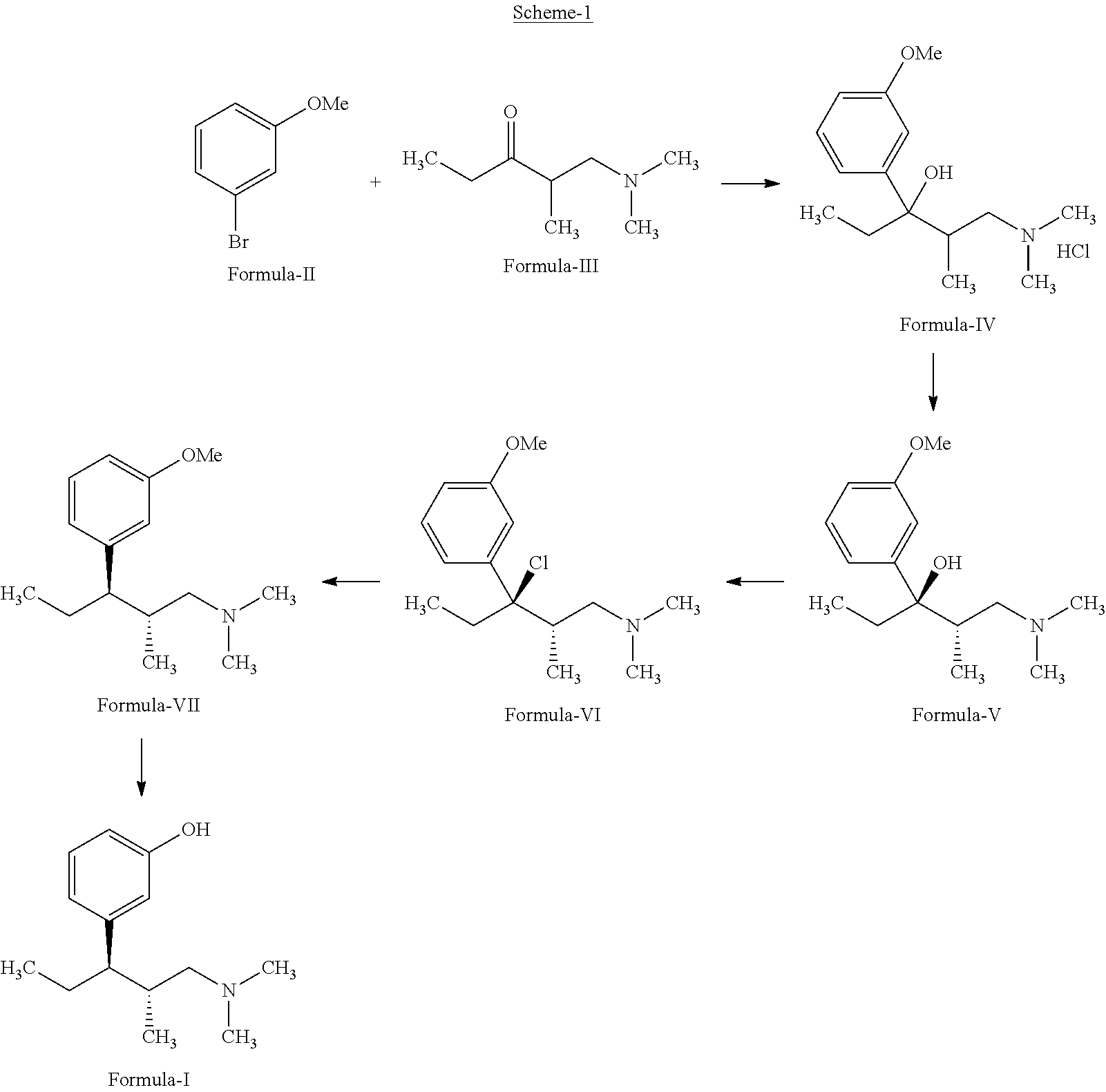
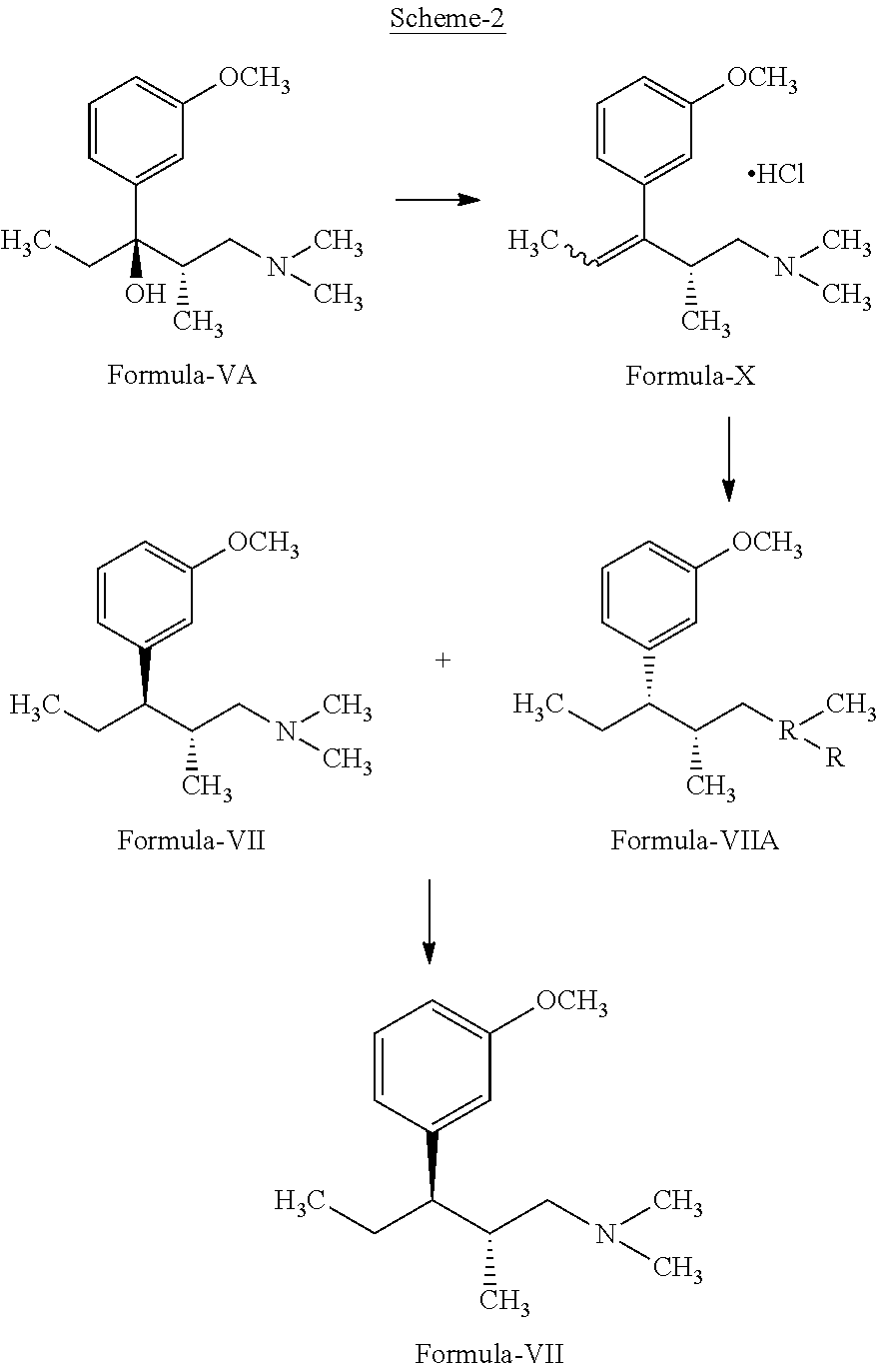
InactiveUS20130137890A1Clean reactionImprove securityAmino preparation from aminesOrganic compound preparationPhenolDeoxygenation
Disclosed herein is an improved process for the preparation of 3-[(2R,3R)-1-(dimethylamino)-2-methylpentan-3-yl]phenol of Formula-I and its pharmaceutically acceptable salt which comprises the reaction of (S)-1-(dimethylamino)-2-methylpentan-3-one of formula VIII with 3-bromo anisole of formula II under Grignard conditions to get the compound (2S, 3R)-1-(dimethylamino)-3-(3-methoxyphenyl)-2-methyl pentan-3-ol of formula V followed by activation of the —OH group of the formula V to convert into sulfonate esters of formula IX, which are on reductive deoxygenation to yield (2R,3R)-3-(3-methoxyphenyl)-N,N,2-trimethylpentan-1-amine of formula VII and demethylation of formula VII to obtain the compound 3-[(2R,3R)-1-(dimethylamino)-2-methylpentan-3-yl]phenol of Formula-1.
Owner:INDOCO REMEDIES
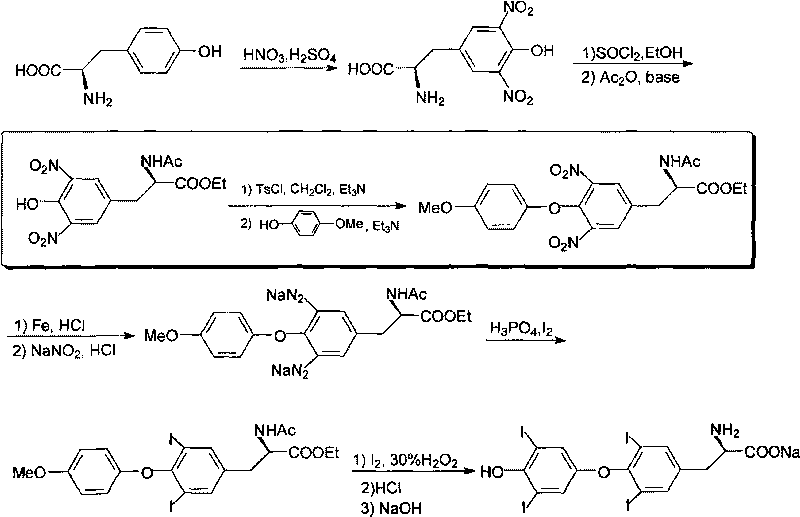
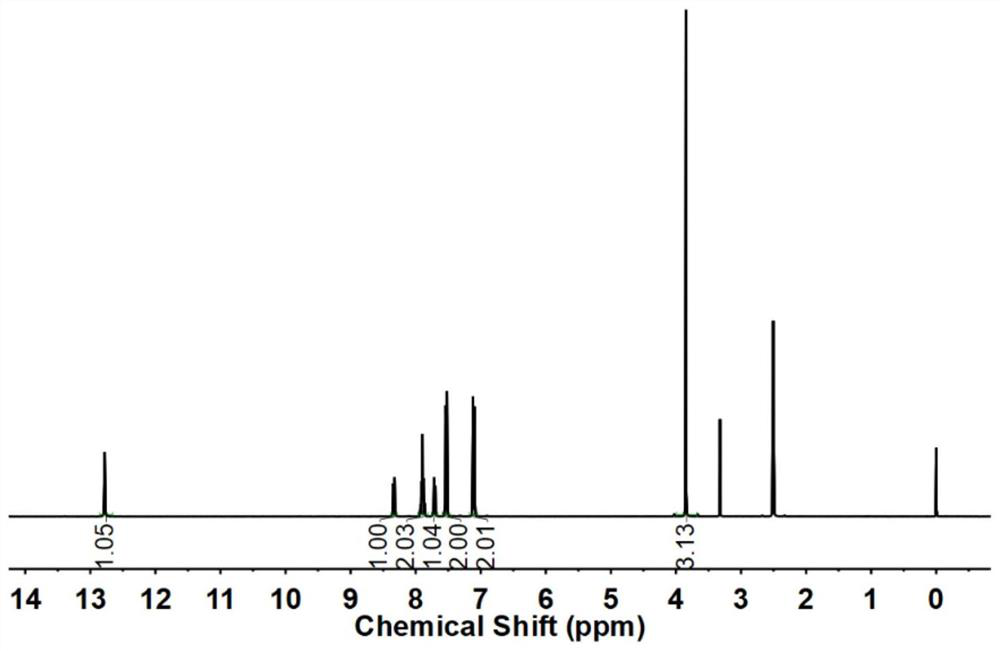
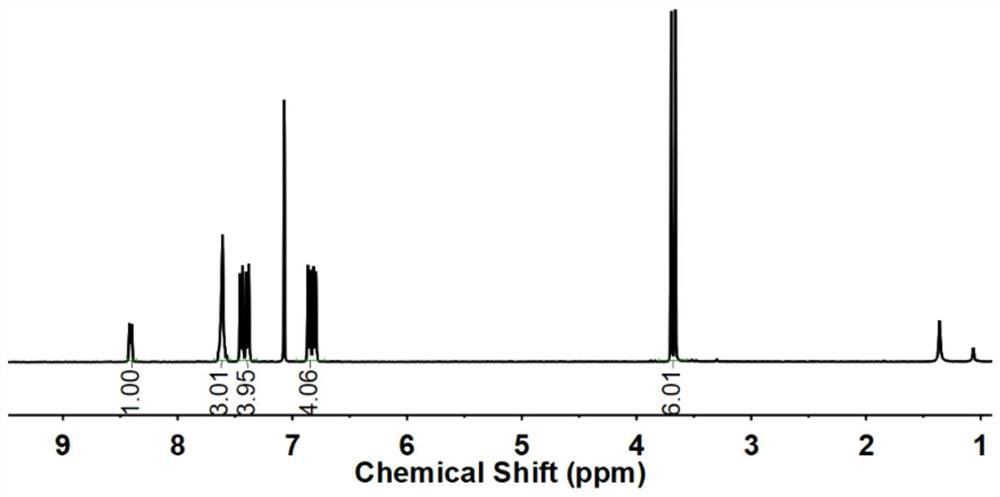
Preparation method of 4-bromoanisole
InactiveCN104230675AHigh yieldEasy to operateOrganic chemistryOrganic compound preparationMeth-Nitrate salts
The invention relates to a preparation method of 4-bromoanisole. The preparation method comprises the following steps: under an oxidizing gas atmosphere, dissolving methoxybenzene and liquid bromine into 1-butyl-3-methylimidazole nitrate, and reacting for more than 1h under closed conditions at 25-100 DEG C to obtain a 4-bromoanisole product. The preparation method disclosed by the invention has the advantages that (1) almost all of bromination reagents and raw materials adopted in the preparation method are transformed into the product, and wastes such as hydrobromic acid and the like can be hardly produced; (2) the preparation method is simple in reaction operation, and adopted solvents are hard to volatilize, so that the volatile pollution of organic solvents can be reduced; and (3) the 4-bromoanisole product prepared by using the preparation method is good in purity and high in raw material conversion rate, so that the product quality can be effectively improved, and the production cost can be reduced.
Owner:HENAN UNIV OF SCI & TECH
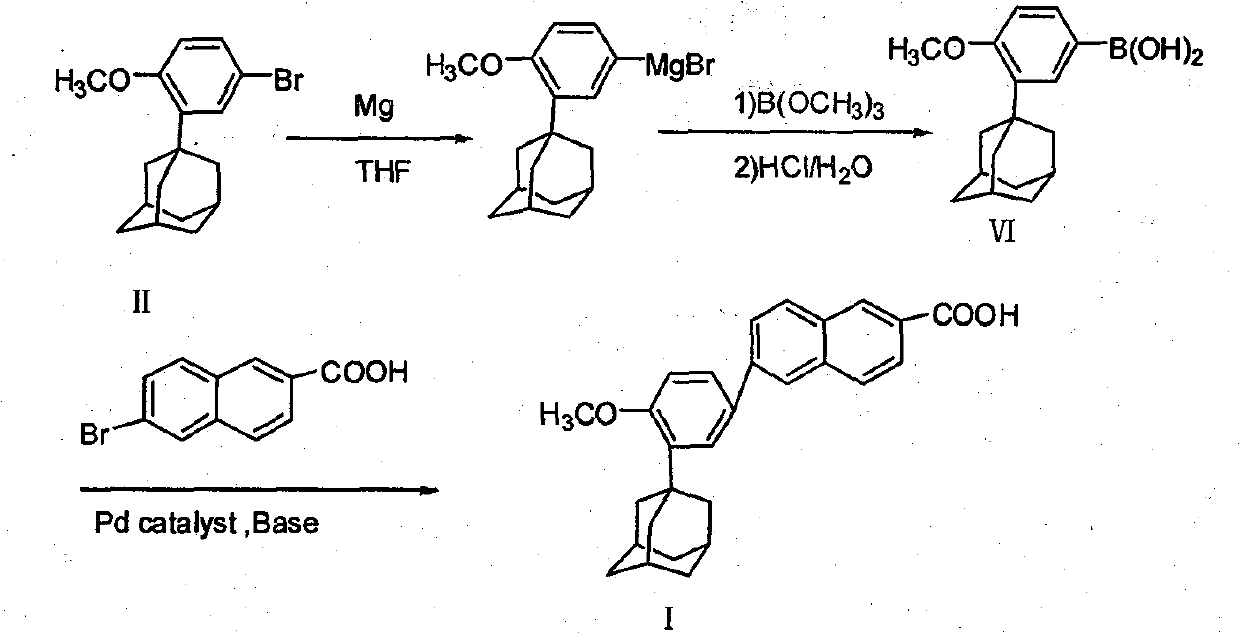
Method of preparing adapalene
InactiveCN101033190AHigh costThorough responseOrganic compound preparationCarboxylic compound preparationSolventBromine
This invention relates to a new method for synthesizing Adapalene 6-[3-(1-King Kong alkyl)-2-naphthoic acid, in which, 2-(1-King Kong alkyl)-4-bromophenyl methyl ether and Zn generate organic Zn reagent in aether-like or non-polarity solvent, which is reacted with 6-bromine-2-naphthoic acid ethyl and analyzed to get the Adapalene.
Owner:北京精华耀邦医药科技有限公司
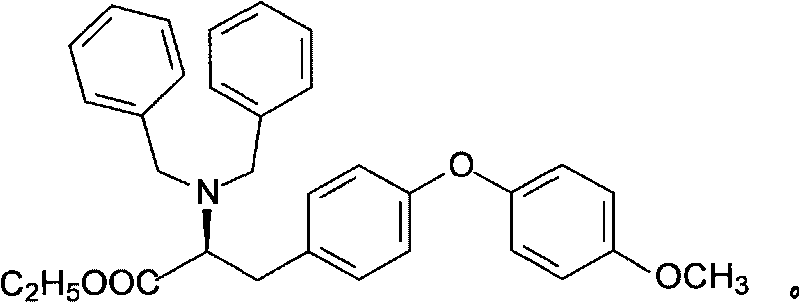
O-p-methoxyphenyl-N,N-ethyl dibenzyl-tyrosine and synthesizing method
InactiveCN101696174ALower reaction costLow costOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsN dimethylformamideTyrosine
The invention discloses an O-p-methoxyphenyl-N,N-ethyl dibenzyl-tyrosine and a synthesizing method. N,N-ethyl dibenzyl-tyrosine and para-bromoanisole are taken as raw materials; under the catalysis of blue vitriod or monohydrate cupric acetate, potassium phosphate is used as alkali, N,N-dimethylglycine hydrochloride is used as a ligand, and coupled reaction is carried out in N,N-dimethylformamide or N-methylpyrrolidone solution at 100 DEG C to obtain the O-p-methoxyphenyl-N,N-ethyl dibenzyl-tyrosine with middling yield and excellent optical purity. In the method, a cuprous salt with poor stability to air and high price is not used as a catalyst, but the potassium phosphate with low price is used as an acid-binding agent, the cheap para-bromoanisole is used as a coupling component, and inert gases for protection and a solvent for purification are not needed during reaction. The preparation method has simple and convenient operation, less equipment requirements, low reaction cost, short process flow and favorable industrialized application prospect and can be developed and used for producing dextrothyroxine sodium.
Owner:ZHEJIANG SCI-TECH UNIV
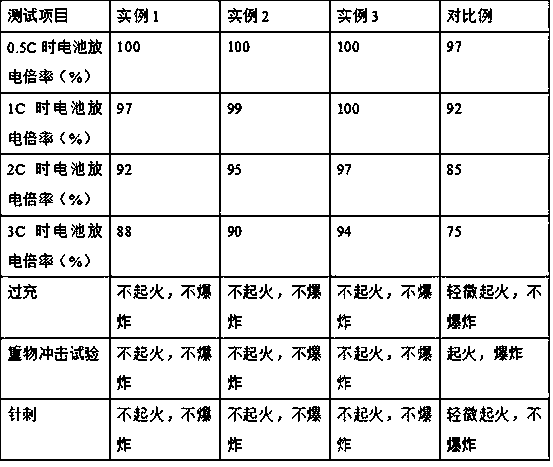
Preparation method of anti-overcharged lithium ion battery pole piece
ActiveCN108258194AAvoid LithiumImprove structural stabilitySecondary cellsElectrode collector coatingInternal resistanceThermal expansion
The invention discloses a preparation method of an anti-overcharged lithium ion battery pole piece and belongs to the technical field of preparation of battery pole pieces. The preparation method disclosed by the invention has the benefits that firstly, sodium dodecyl sulfate is mixed with deionized water to obtain a self-made dispersing liquid, so that the internal resistance of a lithium ion battery is increased, and the side reaction between a negative electrode and an electrolyte is reduced; in addition, particles have high thermal stability; polyethylene, 2-bromoanisole and N-methyl pyrrolidone are subjected to mixed reaction continuously to obtain self-made thermal expansion latex, wherein the polyethylene is a thermal expansion polymer, and once the battery is overcharged or over-discharged, the thermal expansion polymer can quickly expand; in addition, the 2-bromoanisole contains an ortho-bromo group, and the ortho-bromo group increases the stability of a benzene ring Pi bond conjugate system to a certain extent; adsorption products can hinder ion migration, charge transfer and the like, so that the overall impedance property of the battery is increased, and the anti-overcharge and cycle performances of the lithium ion battery pole piece are further improved; therefore, the application prospect is wide.
Owner:北电爱思特(江苏)科技有限公司
Novel process for preparation of 3-bromoanisole and 3-bromonitrobenzene
InactiveCN1296467AImprove performanceOrganic compound preparationEther preparationReaction temperaturePotassium
Process for the preparation of 3-bromoanisole comprising methoxydenitrating 3-bromonitrobenzene in the presence of a phase-transfer catalyst (PTC), and the preparation of 3-bromonirtobenzene by the bromination of nitrobenzene with bromine in oleum. The methoxydenitration reagent in an alkali metal methoxide, which is selected from sodium methoxide and potassium methoxide. The amount of methoxide used is 1-1.5 mol per mol of 3-bromonitrobenzene. The alkali methoxide can be a pre-prepared solid or it can be prepared in situ, by the reaction of the corresponding alkali hydroxide and methanol. In the case when pre-prepared solid methoxide is used, the effective amount of alkali hydroxide is between 1.2-1.7 mol per mol of 3-bromonitrobenzebe. The reaction temperatures are between about 40 to 80° C., with preference to reaction temperatures of 50 to 55° C. In the case in which methoxide is prepared in situ, the effective amount of alkali hydroxide is between 2.2-2.4 mol per mol of 3-bromonitrobenzene. The reaction temperatures are between about 50 to 80° C. with preference to reaction temperatures of 55 to 65° C.
Owner:BROMINE COMPOUNDS
Hetero-naphthalene biphenyl bisphenol monomer and preparation method thereof, hetero-naphthalene biphenyl epoxy monomer and preparation method and application thereof, and flame-retardant epoxy resin
ActiveCN111704581AMild reaction conditionsEasy post-processingOrganic chemistryPolymer sciencePhenanthroline
The invention firstly provides a hetero-naphthalene biphenyl bisphenol monomer and a preparation method thereof. The preparation method comprises the following steps: (1) preparing a unilateral methoxy intermediate MHPZ through a Friedel-Crafts reaction; (2) synthesizing a double-side methoxy intermediate MMPZ by taking MHPZ, p-bromoanisole, 1, 10-phenanthroline and CuI as raw materials; and (3) reducing the MMPZ by using Lewis acid to obtain a bisphenol monomer HHPZ. The synthesis conditions have the advantages of mild reaction conditions, simple post-treatment of the product and high purityof the product. The two ends of the synthesized bisphenol monomer HHPZ are further subjected to epoxidation with epoxy chloropropane, an epoxy monomer with a low melting point and a wide processing window is obtained, the epoxy monomer can be further used for preparing intrinsic flame-retardant epoxy resin, and the flame-retardant grade can reach the V-0 grade.
Owner:DALIAN UNIV OF TECH
A kind of derivative of dibenzofuran and its preparation method and application
ActiveCN105153085BImprove matchImprove thermal stabilityOrganic chemistrySolid-state devicesTert-Butyloxycarbonyl protecting groupPalladium catalyst
The present invention relates to a derivative of dibenzofuran and its preparation method and application. The preparation method of the derivative of dibenzofuran is obtained by coupling p-bromoanisole and p-methoxyaniline through carbon-nitrogen coupling Precursor 1; Precursor 1 and tert-butoxycarbonyl-protected 4,4'-dibromodiphenylamine are reacted in two steps to obtain precursor 2, precursor 1 and tert-butoxycarbonyl-protected 3,6-dibromocarbazole Precursor 3 is obtained through two-step reaction; the above precursor is respectively coupled with 2,8-dibromodibenzofuran under the action of palladium catalyst through carbon-nitrogen coupling to obtain a derivative of dibenzofuran. The derivatives of dibenzofuran prepared by the present invention are easy to synthesize, low in cost, high in glass transition temperature and good in thermal stability, and are excellent hole transport materials, which can be used in perovskite solar cells to obtain Had a good effect.
Owner:VALIANT CO LTD
A kind of synthetic method of crude torene terpenoid skeleton compound
ActiveCN108191803BSimple and fast operationLow costOrganic chemistryChemical synthesisFormylation reaction
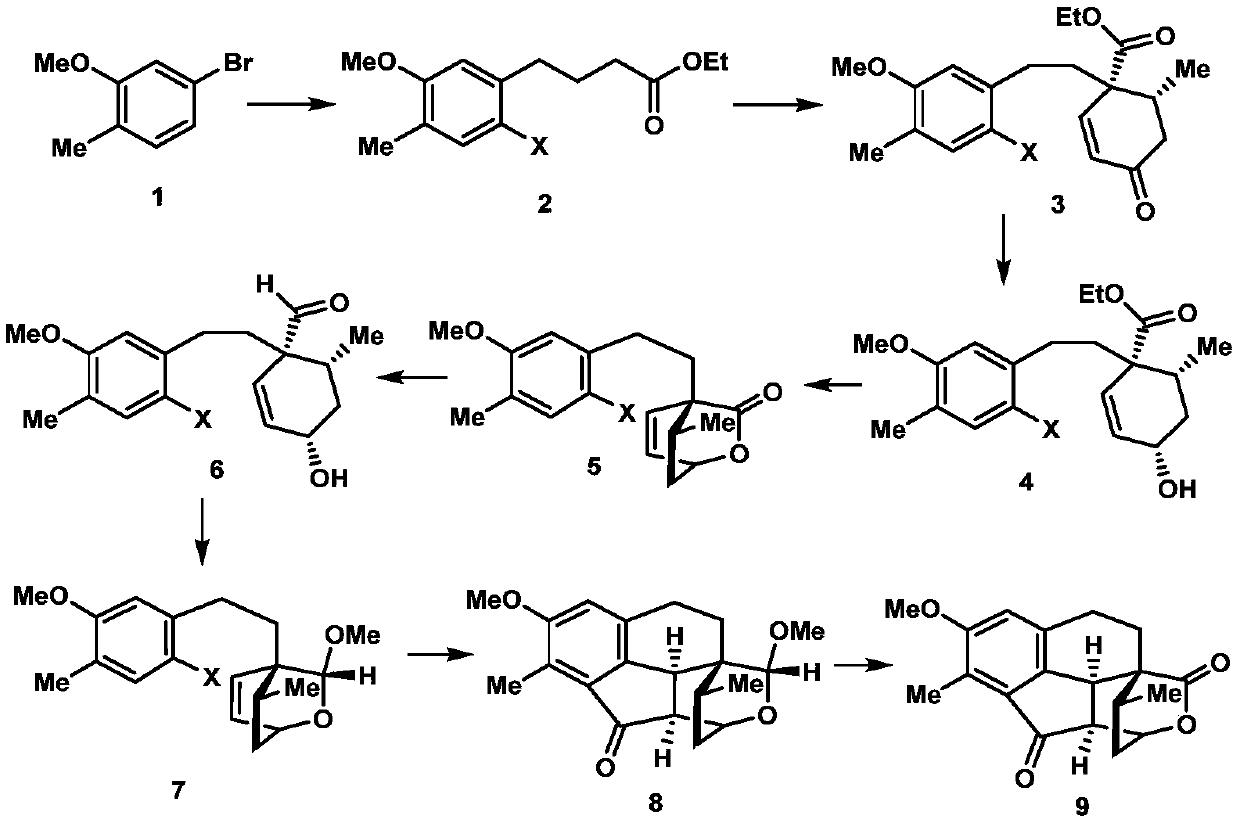
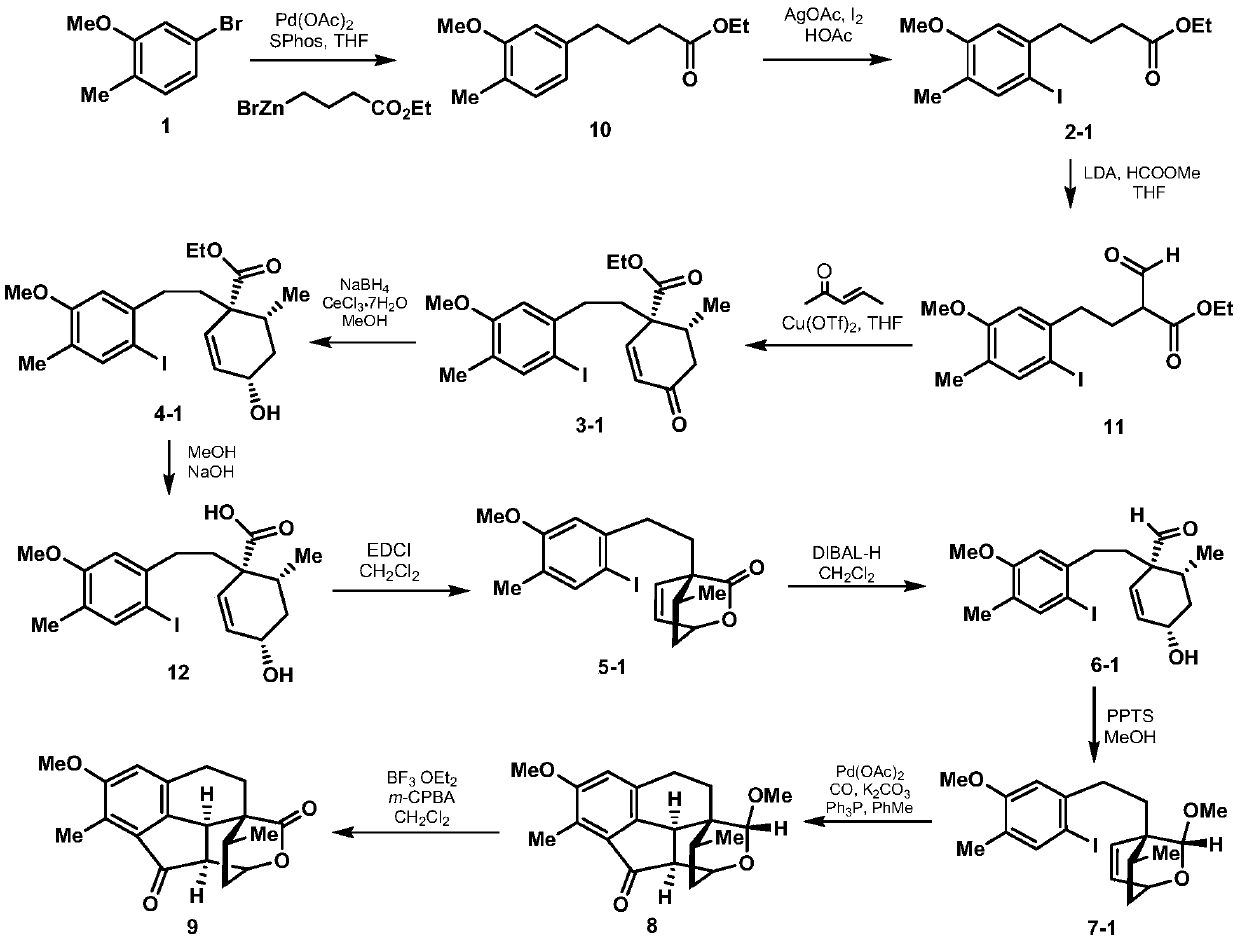
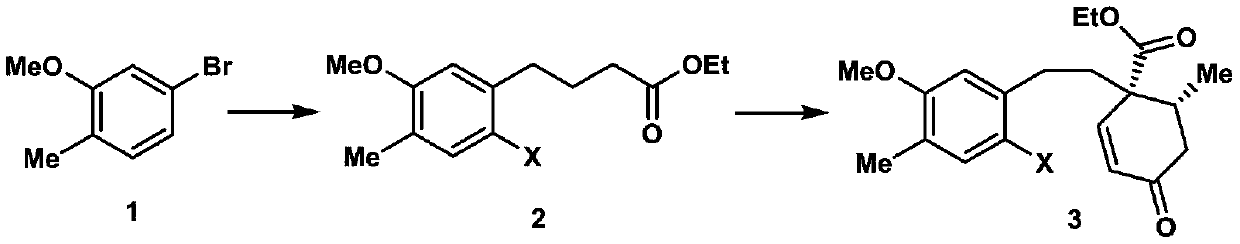
The invention discloses a synthesis method of a cephalotaxus sinensis terpenoid framework compound. The method comprises the following steps: commercially available 2-methyl-5-bromoanisole as a synthetic raw material is subjected to Ei-ichi Negishi reaction for para-halogenation with a methoxyl group, then a product is subjected to formylation reaction with lithium diisopropylamide, and a 1,3-dicarbonyl compound is obtained; the 1,3-dicarbonyl compound is subjected to a Robinson cyclization reaction with 3-pentene-2-one, and a lactone compound is obtained through Luche reduction, hydrolysis and esterification reaction; acid treatment is performed after reduction by diisobutylaluminum hydride, a Heck reaction precursor is obtained and subjected to palladium-catalyzed carbonyl esterificationcoupling reaction, and chemical synthesis of the crude cephalotaxus sinensis terpenoid framework compound is achieved by esterification. The synthetic route has the advantages of being simple, efficient, convenient to operate, low in cost and the like, and the method is applicable to large-scale synthesis of the cephalotaxus sinensis terpenoid framework compound, and provides an important experimental basis for chemical synthesis of cephalotaxus sinensis terpenoid natural products.
Owner:SHAANXI NORMAL UNIV
A kind of preparation method of bisphenol monomer containing phthalazinone structure
The invention discloses a preparation method of a bisphenol monomer containing a phthalazinone structure, belonging to the technical field of new materials. The bisphenol monomer containing phthalazinone structure in the present invention is synthesized by two-step reaction. Using bisphenol-like monomers containing phthalazinone structure and p-bromoanisole as raw materials, using 1,10-phenanthroline as N-N bidentate ligand, CuI as catalyst, through the first step The intermediate product DMPPZ was obtained after C‑O, C‑N Ullmann coupling reaction, and the target monomer DHPPZ was obtained by Lewis acid reduction. The synthesis reaction conditions provided by this patent have the advantages of short synthesis cycle and mild conditions. The purity of the target monomer was tested by liquid mass spectrometry to be 98%, and the yield was 85%-90%.
Owner:DALIAN UNIV OF TECH
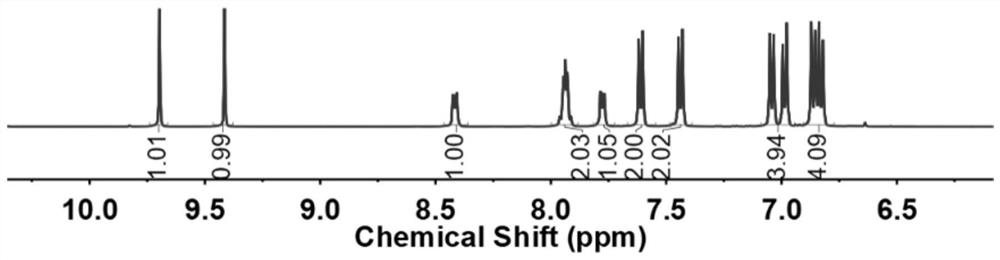
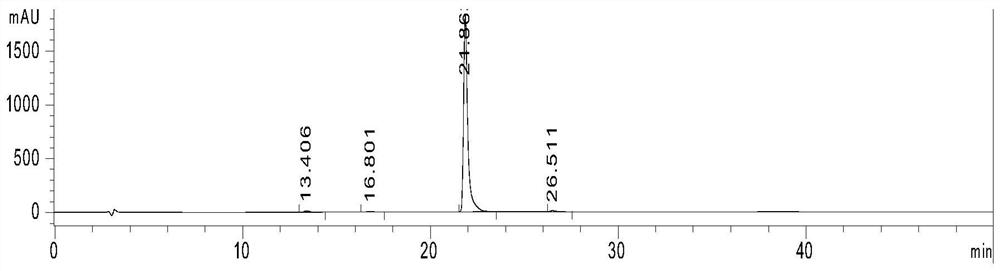
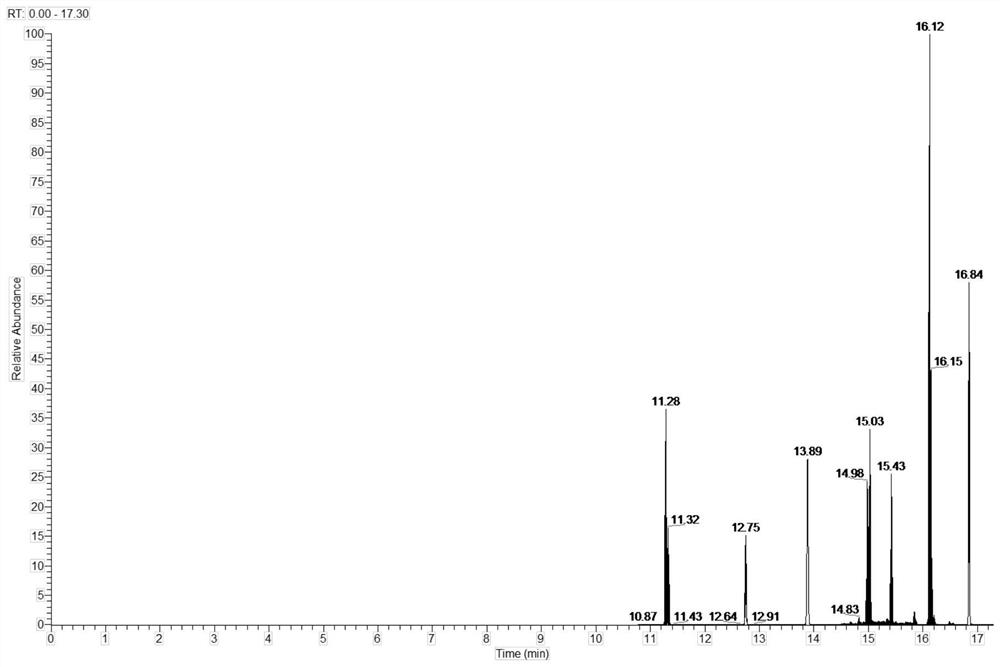
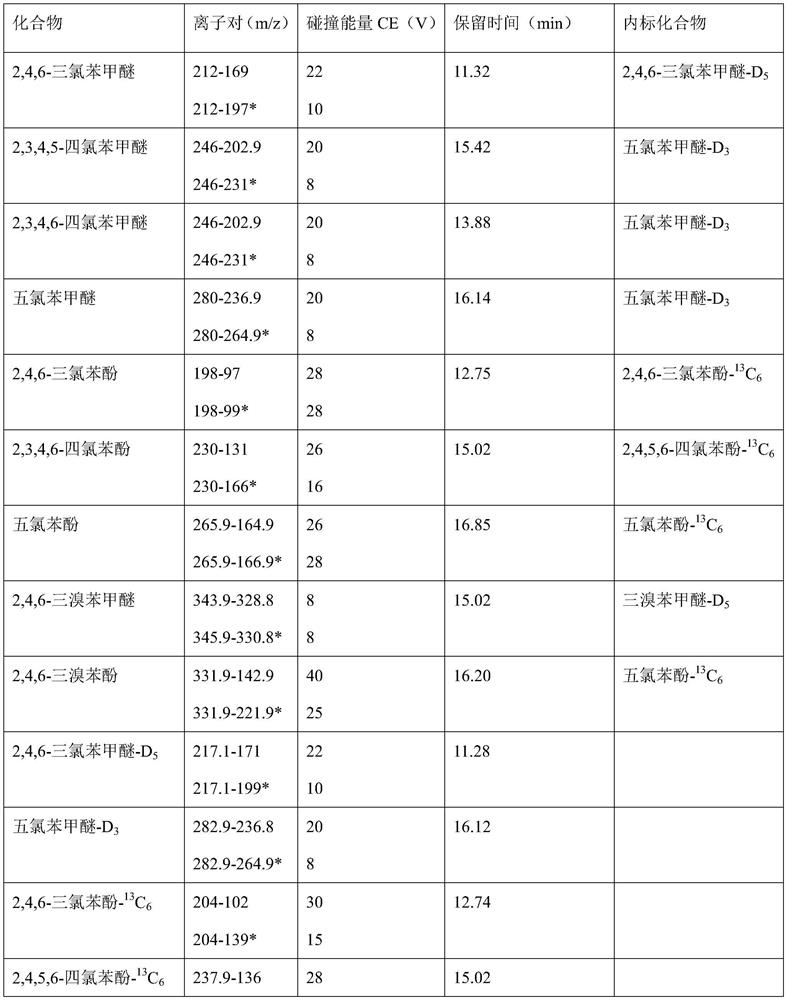
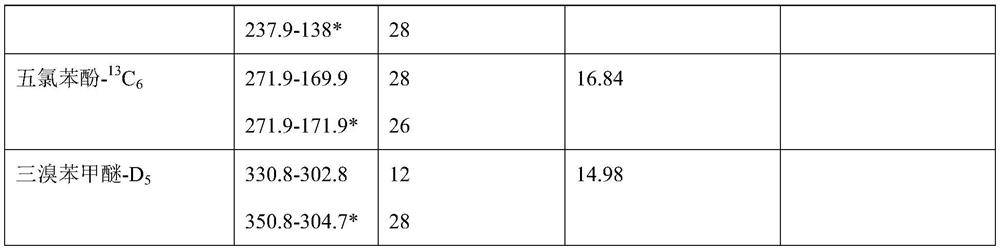
Method for simultaneously determining various wooden plug pollutants in grape wine
The invention discloses a method for simultaneously determining multiple wooden plug pollutants in grape wine, and belongs to the technical field of grape wine detection. The method mainly comprises the following steps: derivatization treatment of a target object and an internal standard, determination of characteristic ions of the target object and the internal standard, establishment of a gas chromatography-tandem mass spectrometry selective reaction monitoring method, determination of a standard curve and detection of the cork pollutants in the grape wine. The method can be used for simultaneously determining nine wooden plug pollutants such as 2, 4, 6-trichloroanisole, 2, 3, 4, 5-tetrachloroanisole, 2, 3, 4, 6-tetrachloroanisole, pentachloroanisole, 2, 4, 6-trichlorophenol, 2, 3, 4, 6-tetrachlorophenol, pentachlorophenol, 2, 4, 6-tribromoanisole and 2, 4, 6-tribromophenol. The detection method is good in linear correlation and standard recovery rate, the detection limit is 0.1 ng / L-0. 5 ng / L, and a technical guarantee can be provided for detection of the cork pollutants in the wine.
Owner:秦皇岛海关技术中心
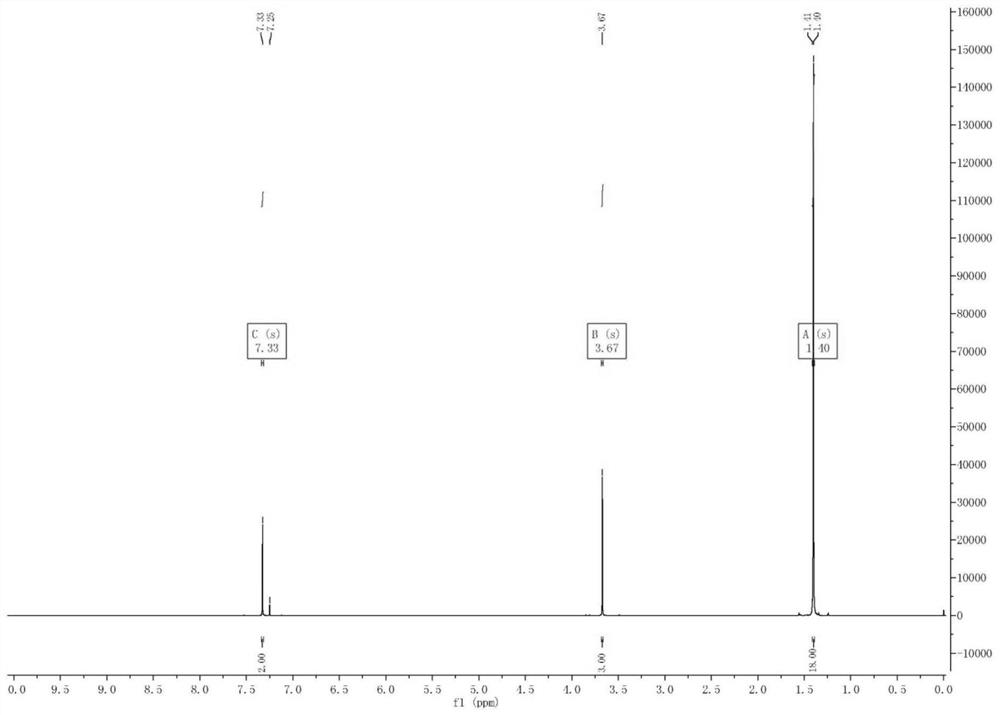
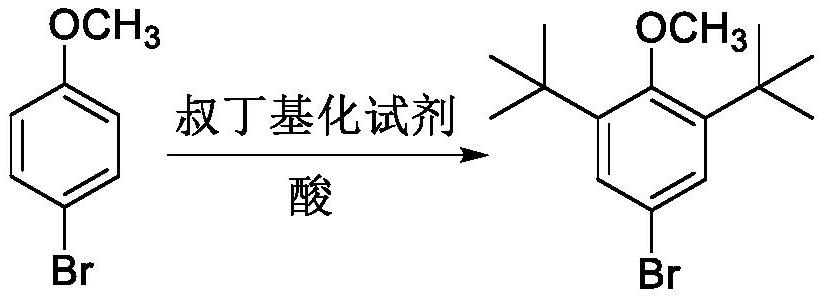
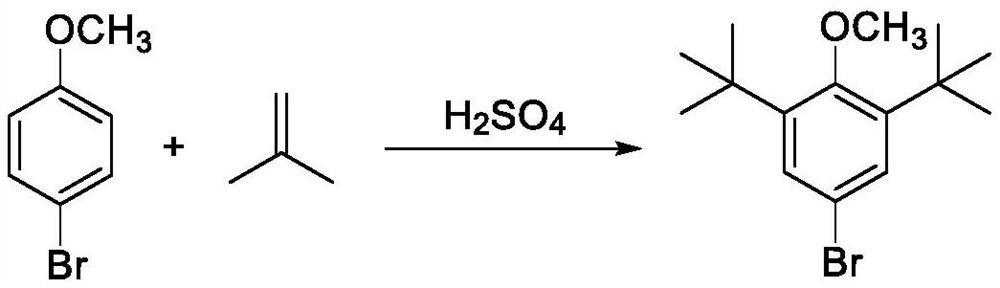
Preparation method of 2, 6-di-tert-butyl-4-bromoanisole
PendingCN113603572ALow toxicityIncrease productivityOrganic chemistryOrganic compound preparationBiochemical engineeringAcid catalysis
The invention provides a preparation method of 2, 6-di-tert-butyl-4-bromoanisole, which comprises the following steps of: adding a tert-butylation reagent into 4-bromoanisole under the catalysis of acid, and reacting to obtain 2, 6-di-tert-butyl-4-bromoanisole. According to the preparation method disclosed by the invention, 4-bromoanisole which is cheap and easy to obtain is taken as a raw material, a solvent is not required to be used, the 2, 6-di-tert-butyl-4-bromoanisole can be obtained only in one step, the preparation method has the advantages of high production efficiency and low production cost, and the raw materials and auxiliary reagents used in the reaction process are relatively low in toxicity and are more environment-friendly.
Owner:黑龙江立科新材料有限公司
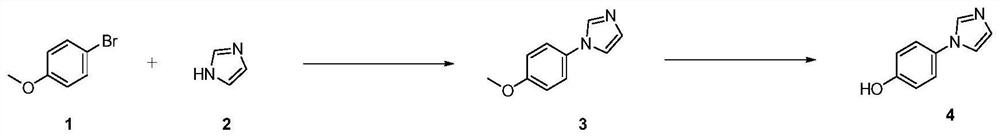
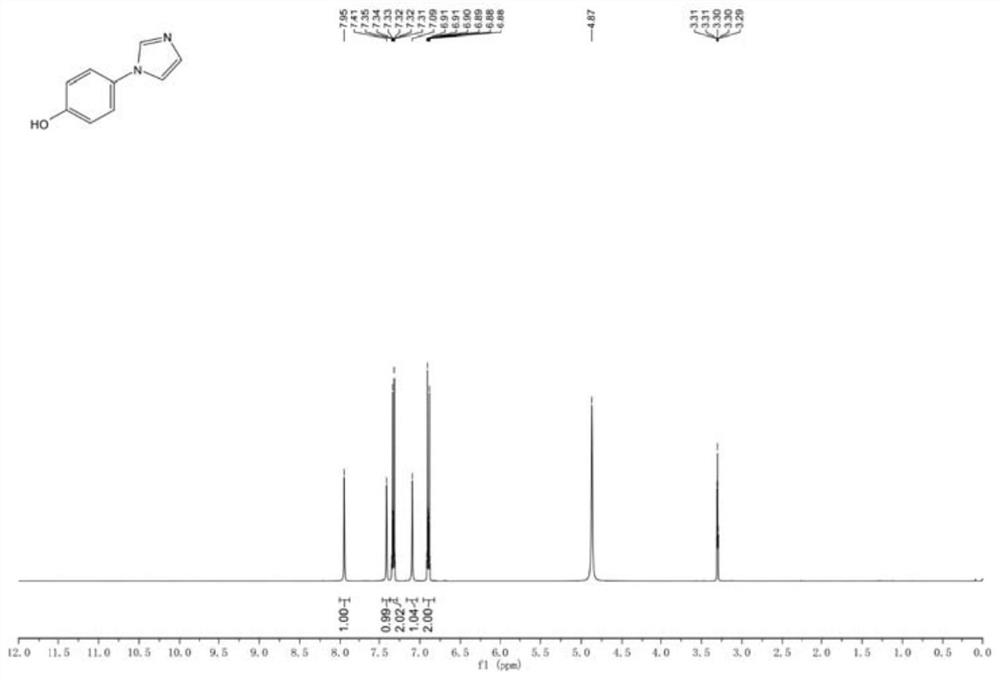
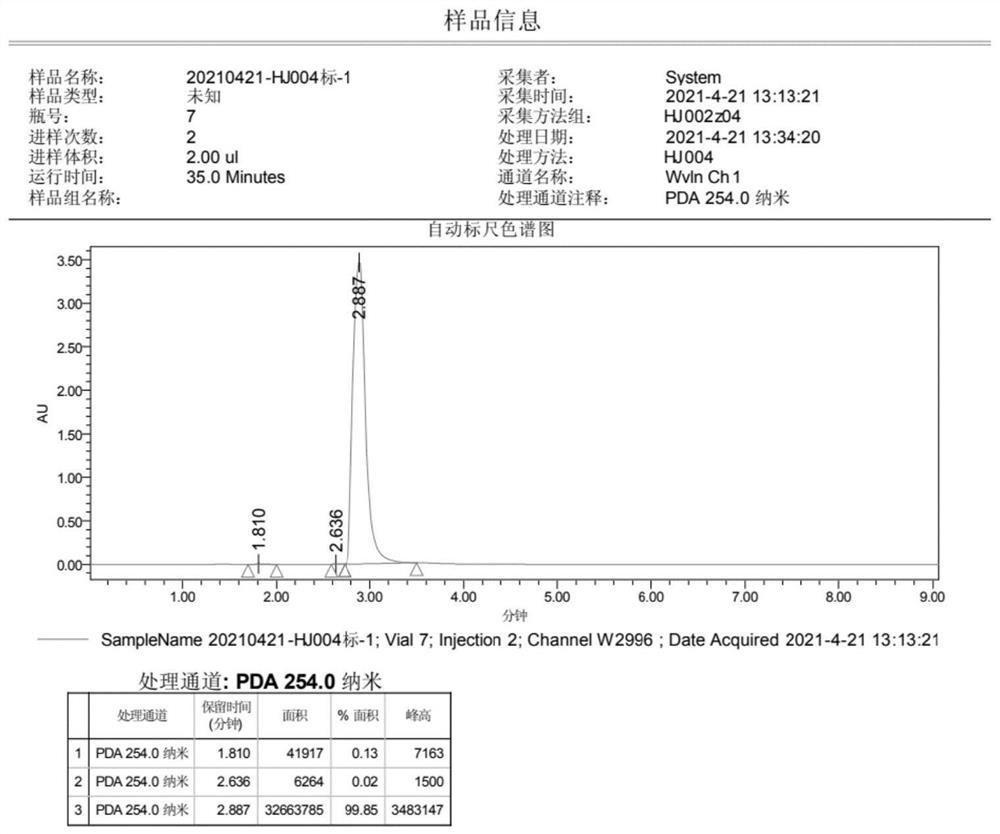
Preparation method of 4-(imidazole-1-yl) phenol
The invention discloses a preparation method of 4-(imidazole-1-yl) phenol, and belongs to the field of in vitro diagnosis. The method comprises the following steps: by taking p-bromoanisole and imidazole as raw materials, carrying out Ullmann reaction in the presence of a catalyst, an alkali and a reaction solvent, extracting to obtain an organic phase after the reaction is finished, adding a saturated saline solution into the organic phase, stirring, separating liquid, drying, and crystallizing by using methyl tert-butyl ether to obtain 1-(4-methoxyphenyl)-1H-imidazole; and carrying out demethylation on the 1-(4-methoxyphenyl)-1H-imidazole by using boron tribromide, and recrystallizing to obtain the 4-(imidazole-1-yl) phenol. According to the method, the post-treatment process is simple, a large amount of solvent extraction and column chromatography purification are not needed, the product can be obtained only through recrystallization, and the prepared product is free of copper ion residues, white in appearance and capable of better meeting the market requirement.
Owner:上海瀚诺威生物科技有限公司
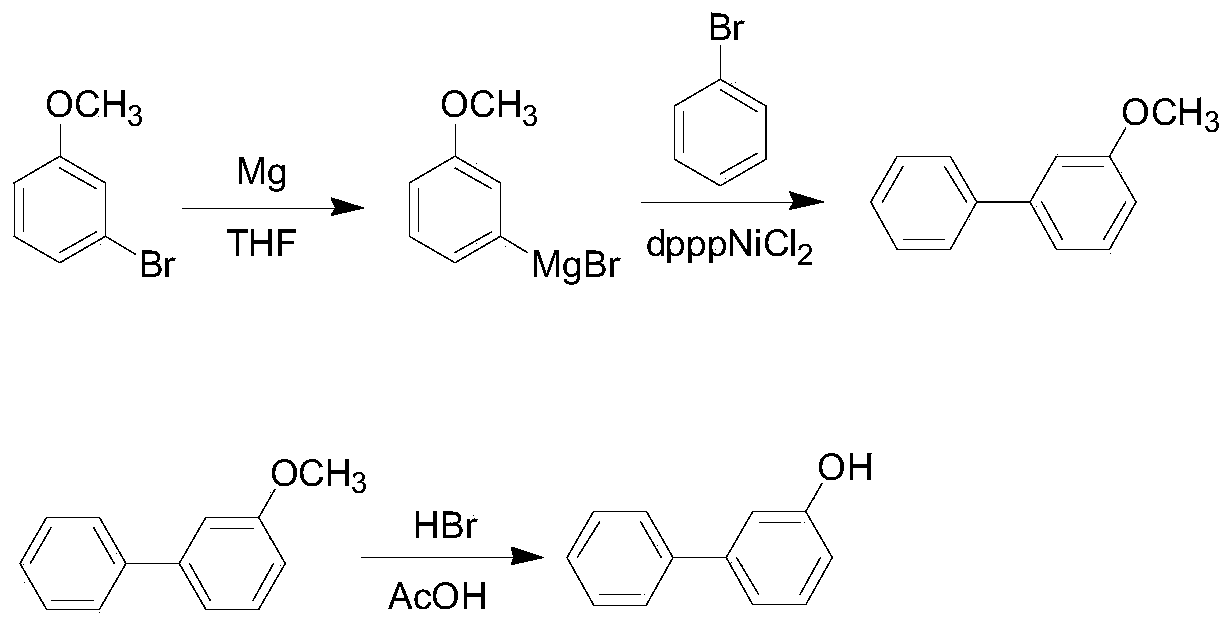
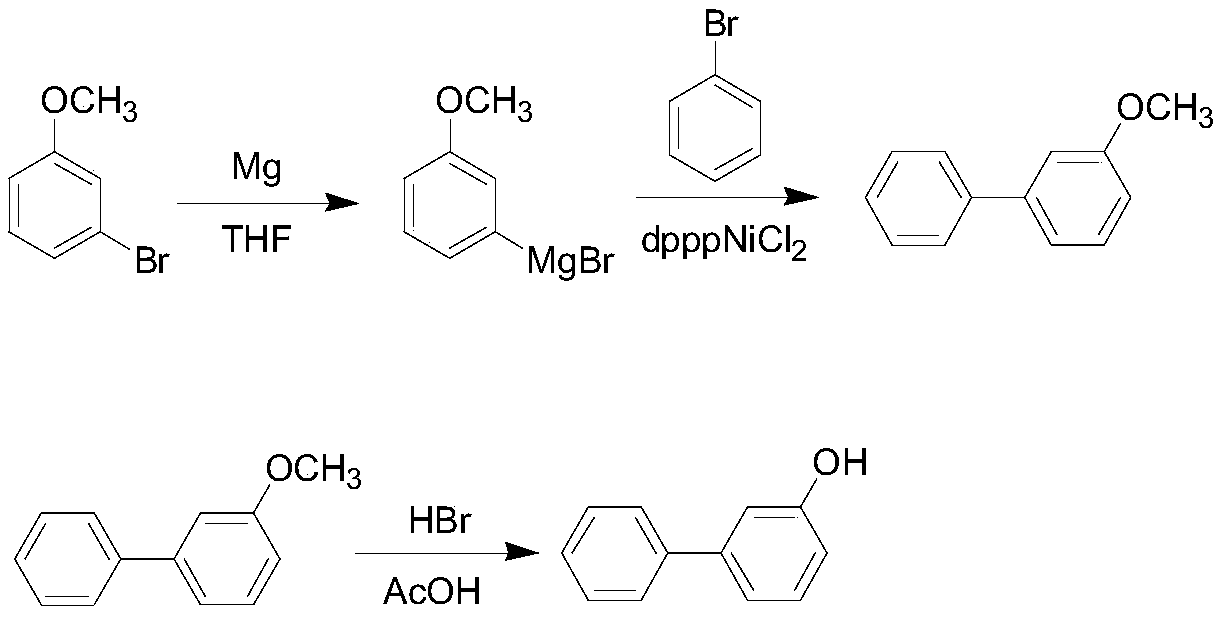
Method for synthesizing 3-hydroxy biphenyl
InactiveCN109796312AMild reaction conditionsEasy to operateOrganic compound preparationMagnesium organic compoundsAcetic acidGrignard reagent
The invention discloses a method for synthesizing 3-hydroxy biphenyl, which is characterized by comprising the following steps of: under the protection of inert gas or N2 gas, using THF as a solvent,reacting bromoanisole with magnesium scraps to obtain a Grignard reagent; reacting Grignard reagent with bromobenzene in the presence of a catalyst dpppNiCl2, adding an acid solution for quenching reaction, layering, extracting water phase, separating liquid, combining the obtained organic phases, washing, drying, concentrating, and distilling to obtain an intermediate 3-methoxybiphenyl; adding the 3-methoxybiphenyl into glacial acetic acid, adding a 48% HBr solution, heating, refluxing, aging, cooling, extracting and separating, mixing the obtained organic phases, washing, drying, concentrating, and recrystallizing toluene to obtain the 3-hydroxy biphenyl. The method has the advantages of convenient operation, easy control of reaction and simple requirement on reaction equipment, the whole process is suitable for industrial production, and the GC purity of the prepared 3-hydroxy biphenyl can reach more than 99%, and the total yield is more than 60%.
Owner:SHANGHAI JINGCHUN BIOCHEM TECH CO LTD
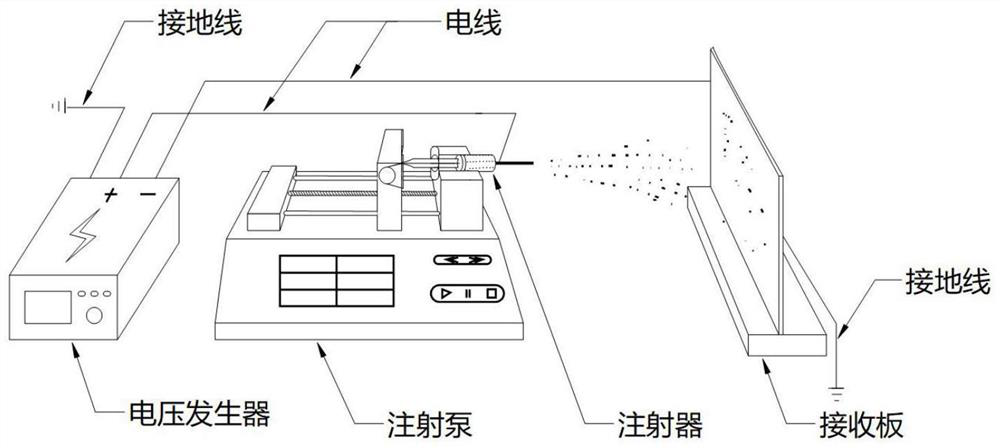
Preparation method of TNBA/TNAZ eutectic mixture
ActiveCN114685227AReduce heatingEasy to operateNitrated acyclic/alicyclic/heterocyclic amine explosive compositionsExplosive ingredient compoundingVoltage generatorMetallurgy
The invention provides a preparation method of a TNBA / TNAZ eutectic mixture. Comprising the following steps: step 1, preparing a solution: grinding 2, 4, 6-trinitro-3-bromoanisole and 1, 3, 3-trinitroazetidine by using a mortar, pouring into an organic solvent, and stirring to prepare a precursor solution; and 2, electrostatic spraying: extracting the precursor solution by using an injector, placing the precursor solution in an injection pump, connecting with a voltage generator, receiving a product by using tin foil paper, and drying to obtain the product. The electrostatic spraying method is adopted, the process is simple and easy to operate, and the morphology and the particle size of the eutectic particles can be controlled by adjusting electrostatic spraying parameters. The eutectoid prepared by the method is relatively good in performance consistency, uniform in particle size distribution and relatively narrow in particle size distribution and can reach the nanoscale, and the phenomena that the performance of different batches of eutectoid prepared by a traditional melting method is inconsistent and the particle size is non-uniform when a solvent anti-solvent method is used for preparing the eutectoid are effectively avoided.
Owner:ZHONGBEI UNIV
Method of preparing adapalene
InactiveCN101033190BNot easy to hydrolyzeHigh yieldOrganic compound preparationCarboxylic compound preparationReaction temperature6-bromo-2-naphthoic acid
This invention relates to a new synthesis route and method of adapalene, namely 6-[3-(1-adamanty)-4-methoxy phenyl]-2-naphthoic acid. In a ethers solvent or a non-polar solvent, an organic zincon is generated by reacting 2-(1-adamanty)-4-bromoanisole with a metal zinc. In the presence of a catalyst of niCl2 / DPPE, the organic zincon is reacted with 6-bromo-2-naphthoic acid methyl ether, then the adapalene is obtained after processes of alkaline saponification reaction, water washing, acidifying with hydrochloric acid and recrystallization or the like. The invention has advantages of mild reaction temperature, simple operation, low cost and high yield, so that the yield of each step is more than 90% and the total yield is more than 65%.
Owner:北京精华耀邦医药科技有限公司
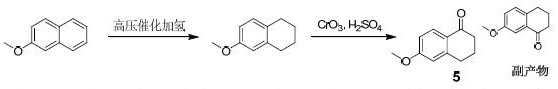
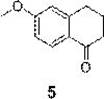
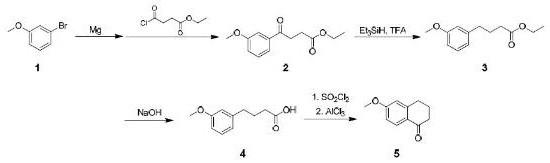
Synthesis method of 6-methoxy-1-tetralone
ActiveCN114436790AOrganic compound preparationCarboxylic acid esters preparationTetraloneButanedioic acid
The invention relates to a synthesis method of 6-methoxy-1-tetralone, which comprises the following steps: 1) adding magnesium metal into toluene, adding iodomethane for initiation, and dropwise adding a toluene solution of 3-bromoanisole to prepare a 3-methoxyphenyl magnesium bromide solution; dissolving ethyl succinate monoacyl chloride in toluene, cooling to-45 DEG C to-55 DEG C, adding a 3-methoxyphenyl magnesium bromide solution, and reacting to prepare a crude product of a compound (2); 2) dropwise adding trifluoroacetic acid (TFA) into the compound (2) and triethyl silane in dichloromethane, and dropwise adding water to obtain an oily compound (3) crude product; 3) dissolving the compound (3) in methanol, adding sodium hydroxide and water, and carrying out a reflux reaction for 3-5 h to obtain a compound (4), and 4) dissolving the compound (4) in dichloromethane, dropwise adding thionyl chloride, and after the reaction is completed, adding anhydrous aluminum trichloride, and after the reaction is completed, carrying out a reaction to obtain the 6-methoxy-1-tetralone.
Owner:ZHEJIANG XIANJU JUNYE PHARM CO LTD +1
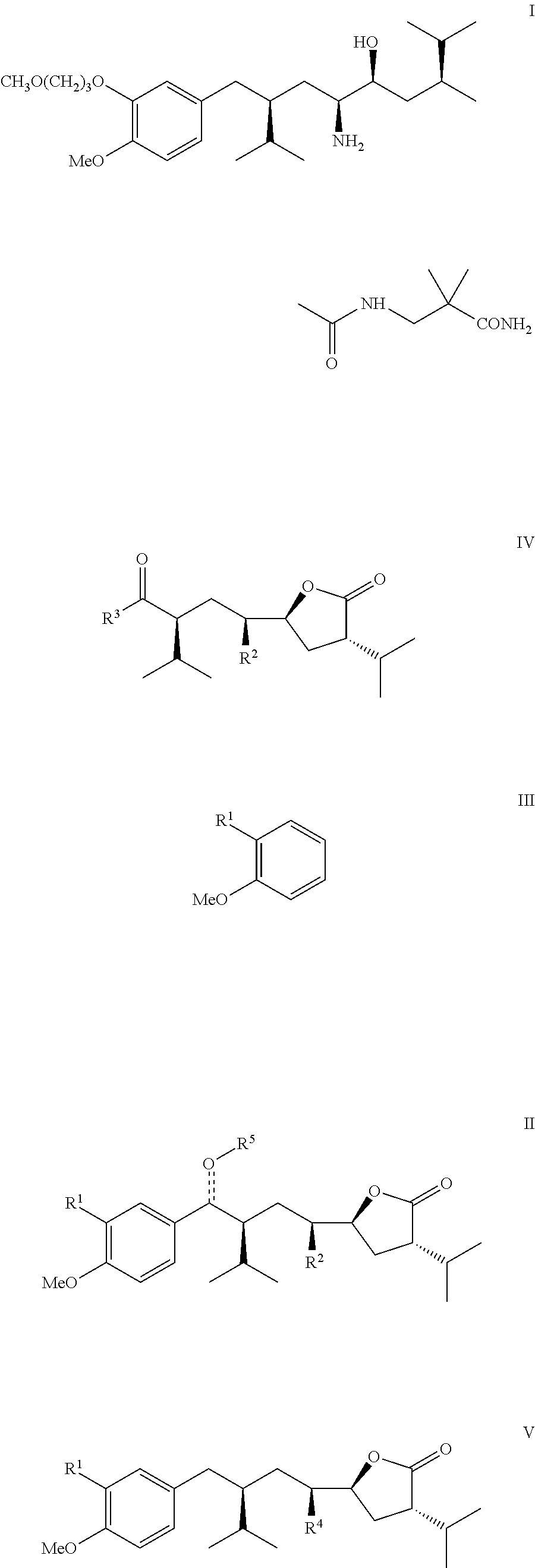
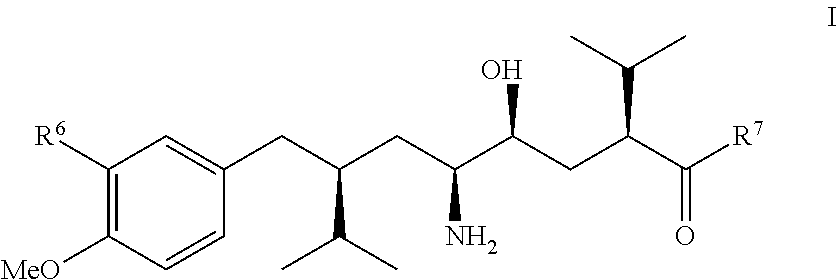
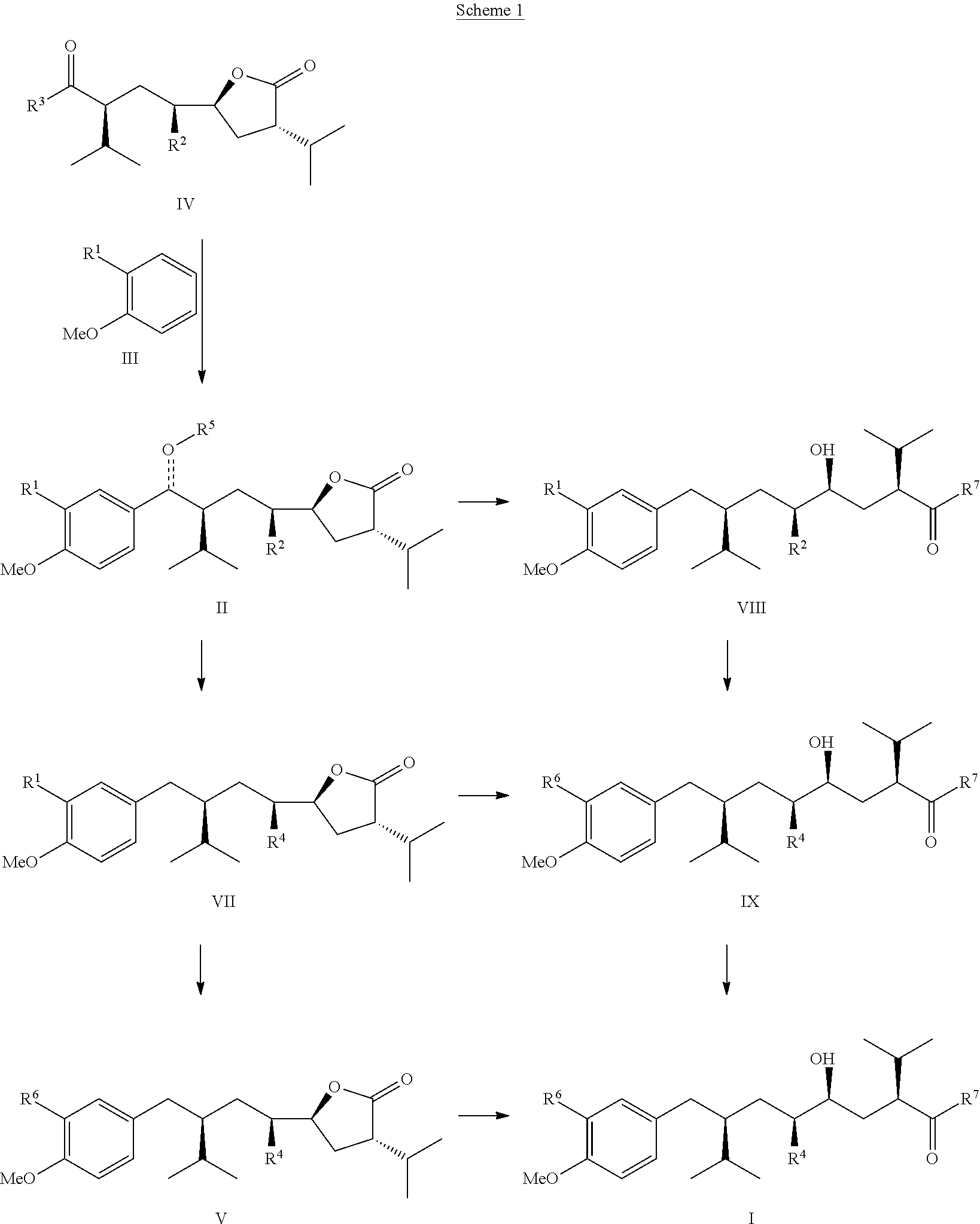
Manufacturing process for 8-aryloctanoic acids such as Aliskiren
The present invention relates to a novel manufacturing process and novel intermediates useful in the synthesis of pharmaceutically active compound such as Aliskiren.The invention describes preparation of enantiomerically pure 8-aryloctanoic acid of formula I from a chiral compound of formula IV. Friedel-Crafts reaction of this compound of formula IV with a compound of formula III provides compound of formula II which is converted reductively in a few steps into the compound of formula V, a know intermediate in the synthesis of compound of formula I. According to the disclosed process, Aliskiren can be now prepared from commercial starting materials (Guajacol or 2-bromoanisole, cis- or trans-1,4-dichloro-2-butene and 4(S)-benzyl-3-isovaleroyl-oxazolidin-2-one) in less than 8 process steps.
Owner:MILAN SOUKUP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Process for preparing 6-[3-(1-adamantyl)-4-methoxy phenyl]-2-methyl naphthoate Process for preparing 6-[3-(1-adamantyl)-4-methoxy phenyl]-2-methyl naphthoate](https://images-eureka.patsnap.com/patent_img/0d52ea28-cf24-44e2-b862-496a5159046b/A2006100396160002C1.PNG)
![Process for preparing 6-[3-(1-adamantyl)-4-methoxy phenyl]-2-methyl naphthoate Process for preparing 6-[3-(1-adamantyl)-4-methoxy phenyl]-2-methyl naphthoate](https://images-eureka.patsnap.com/patent_img/0d52ea28-cf24-44e2-b862-496a5159046b/A20061003961600031.PNG)
![Process for preparing 6-[3-(1-adamantyl)-4-methoxy phenyl]-2-methyl naphthoate Process for preparing 6-[3-(1-adamantyl)-4-methoxy phenyl]-2-methyl naphthoate](https://images-eureka.patsnap.com/patent_img/0d52ea28-cf24-44e2-b862-496a5159046b/A20061003961600032.PNG)
![Process for preparing 2-[(dimethylamino)-methyl]-1-(3-methoxyphenyl)cyclohexanol Process for preparing 2-[(dimethylamino)-methyl]-1-(3-methoxyphenyl)cyclohexanol](https://images-eureka.patsnap.com/patent_img/3d260a78-286e-4a6c-a153-70eee2ffeeaa/US07030276-20060418-C00001.png)
![Process for preparing 2-[(dimethylamino)-methyl]-1-(3-methoxyphenyl)cyclohexanol Process for preparing 2-[(dimethylamino)-methyl]-1-(3-methoxyphenyl)cyclohexanol](https://images-eureka.patsnap.com/patent_img/3d260a78-286e-4a6c-a153-70eee2ffeeaa/US07030276-20060418-C00002.png)
![Process for preparing 2-[(dimethylamino)-methyl]-1-(3-methoxyphenyl)cyclohexanol Process for preparing 2-[(dimethylamino)-methyl]-1-(3-methoxyphenyl)cyclohexanol](https://images-eureka.patsnap.com/patent_img/3d260a78-286e-4a6c-a153-70eee2ffeeaa/US07030276-20060418-C00003.png)
![Process for preparing 2-[(dimethylamino)-methyl]-1-(3-methoxyphenyl)cyclohexanol Process for preparing 2-[(dimethylamino)-methyl]-1-(3-methoxyphenyl)cyclohexanol](https://images-eureka.patsnap.com/patent_img/d6159b96-d8c6-4777-923a-5b3d9a160831/US20050215821A1-20050929-C00001.png)
![Process for preparing 2-[(dimethylamino)-methyl]-1-(3-methoxyphenyl)cyclohexanol Process for preparing 2-[(dimethylamino)-methyl]-1-(3-methoxyphenyl)cyclohexanol](https://images-eureka.patsnap.com/patent_img/d6159b96-d8c6-4777-923a-5b3d9a160831/US20050215821A1-20050929-C00002.png)
![Process for preparing 2-[(dimethylamino)-methyl]-1-(3-methoxyphenyl)cyclohexanol Process for preparing 2-[(dimethylamino)-methyl]-1-(3-methoxyphenyl)cyclohexanol](https://images-eureka.patsnap.com/patent_img/d6159b96-d8c6-4777-923a-5b3d9a160831/US20050215821A1-20050929-C00003.png)