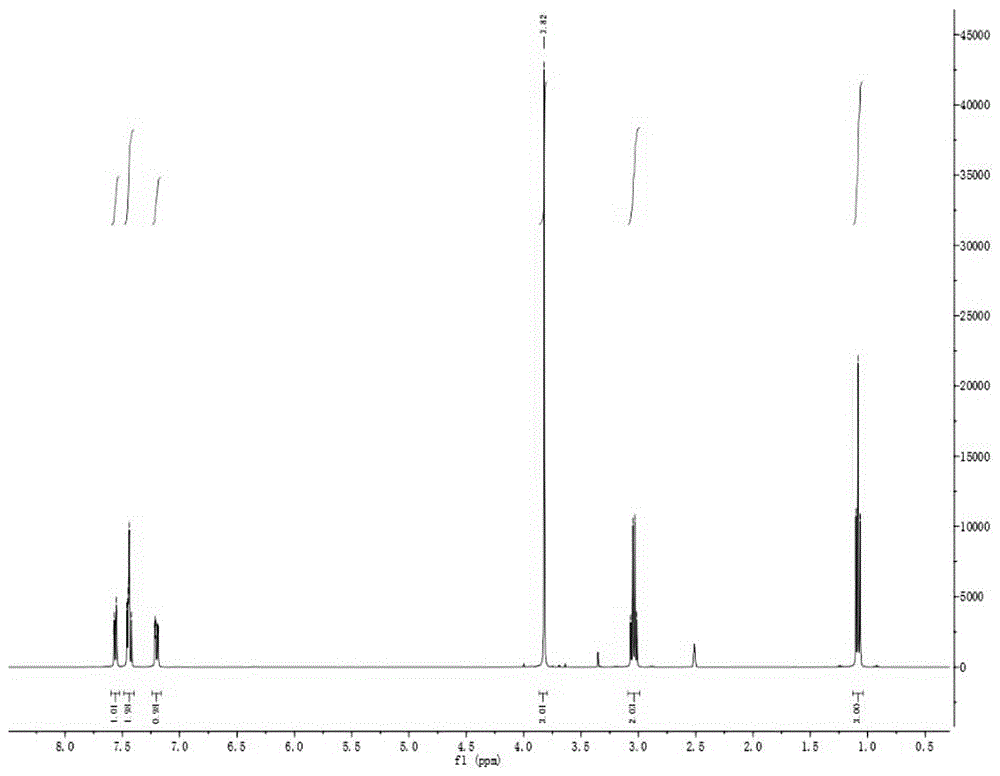
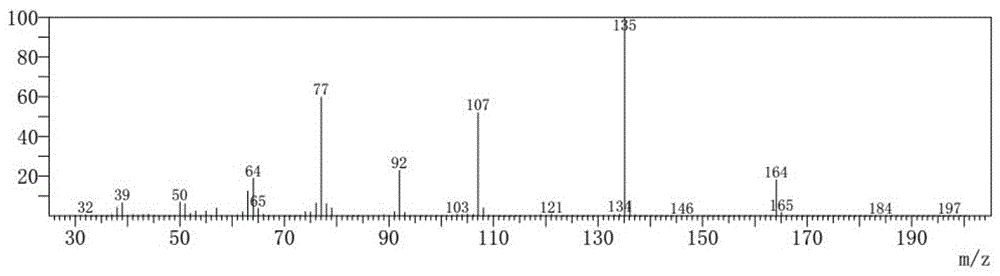
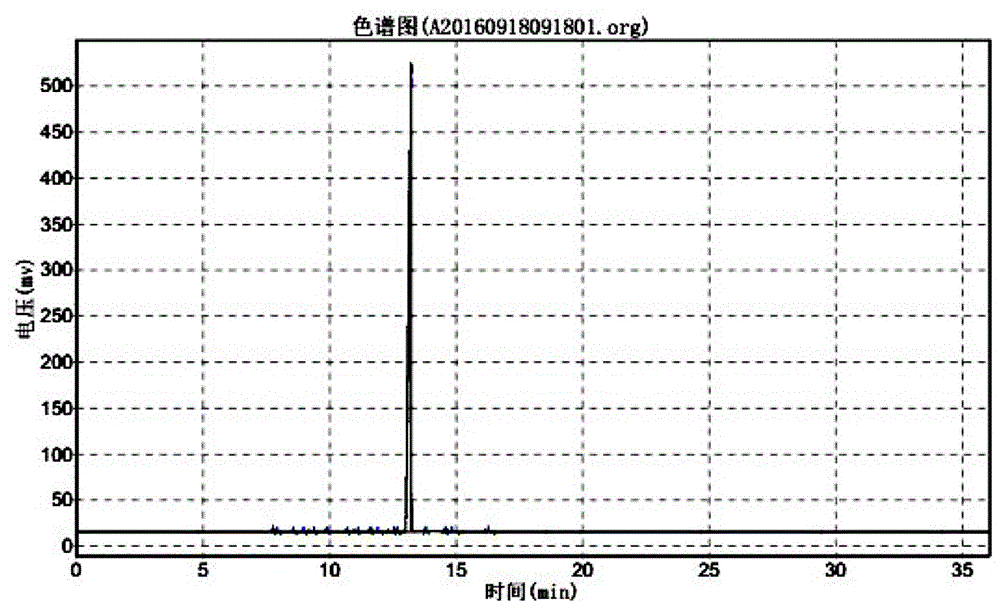
Synthesis method for 3-methoxypropiophenone
A technology of methoxybenzene and synthesis method, which is applied in the field of synthesis of pharmaceutical intermediate compounds, can solve the problems of low yield and high cost, and achieve the effects of simple operation, low cost and advanced technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 24.0 g (1.0 mol) of magnesium powder, 3.0 g of anhydrous aluminum trichloride, and 300 mL of tetrahydrofuran (THF) solution into a reaction flask equipped with a reflux condenser and a dropping funnel, and heat. Slowly add a mixed solution of 187.1 g (1.0 mol) of m-methoxy bromobenzene and 300 mL of tetrahydrofuran (THF) into the dropping funnel, and control the temperature of the reaction solution to 50-55°C to keep the solution in a slightly boiling state. After the addition, heat Reflux for 0.5-1.0h to ensure complete magnesium reaction and obtain Grignard reagent. Under stirring, 55.1 g (1.0 mol) of propionitrile was slowly added to the Grignard reagent, and after the addition was completed, the reaction was carried out for another 1.0-2.0 h. After the reaction is completed, add 3.0 mol / L hydrochloric acid dropwise slowly under a cold water bath to decompose the addition product and separate the inorganic phase. The organic phase is distilled off under normal pre...
Embodiment 2
[0026] The amount of m-methoxy bromobenzene was increased to 205.7 g (1.1 mol), propionitrile was 60.5 g (1.1 mol), and others were the same as in Example 1, and the yield of 3-methoxypropiophenone was 82.5%.
Embodiment 3
[0028] The amount of m-methoxy bromobenzene was increased to 224.5 g (1.2 mol), propionitrile was 66.1 g (1.2 mol), and the other same as in Example 1, the yield of 3-methoxypropiophenone was 85.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com