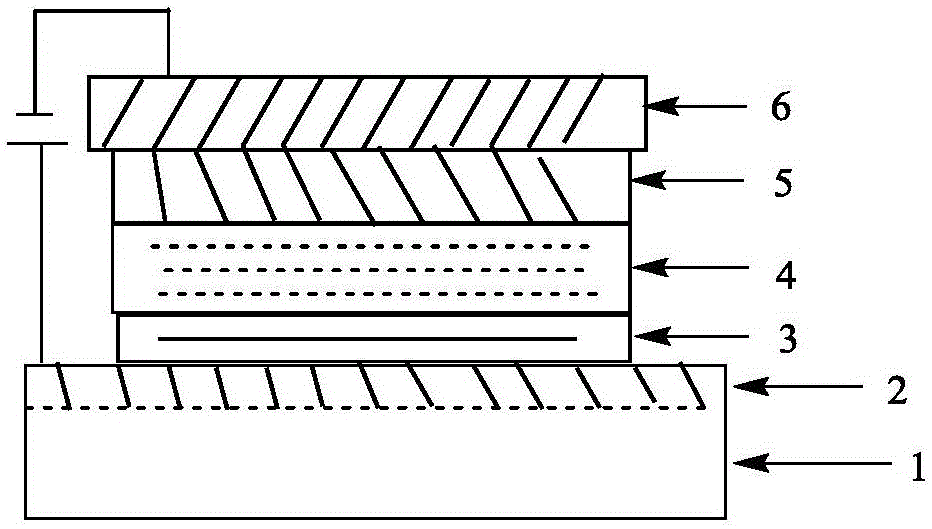
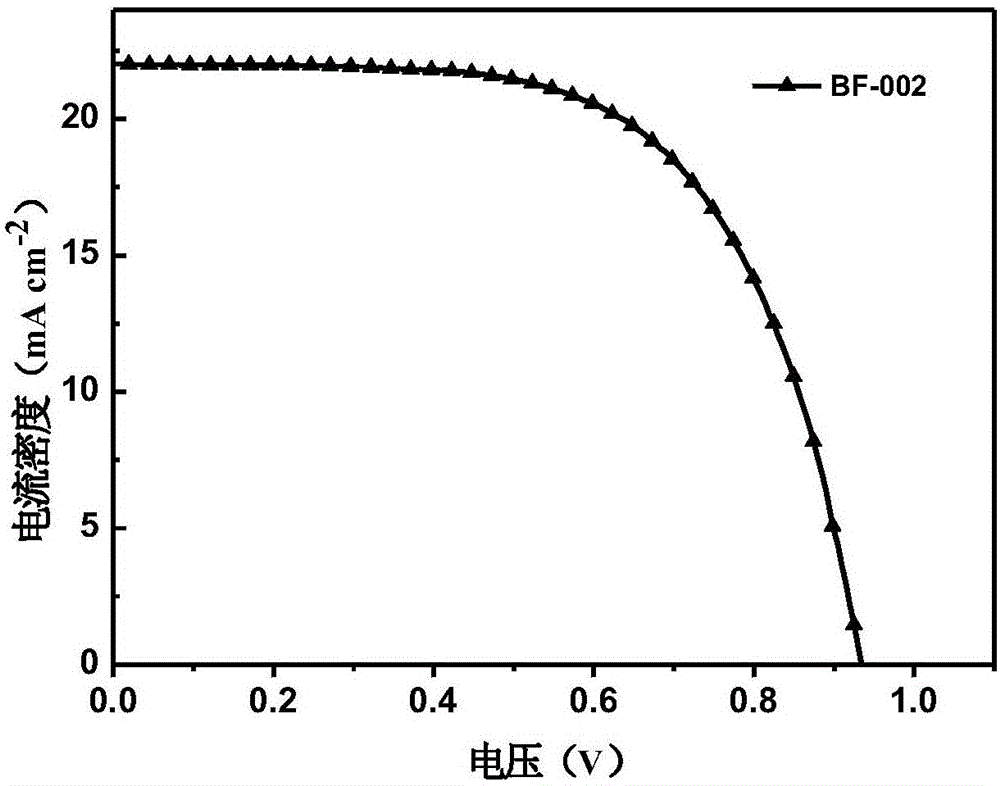
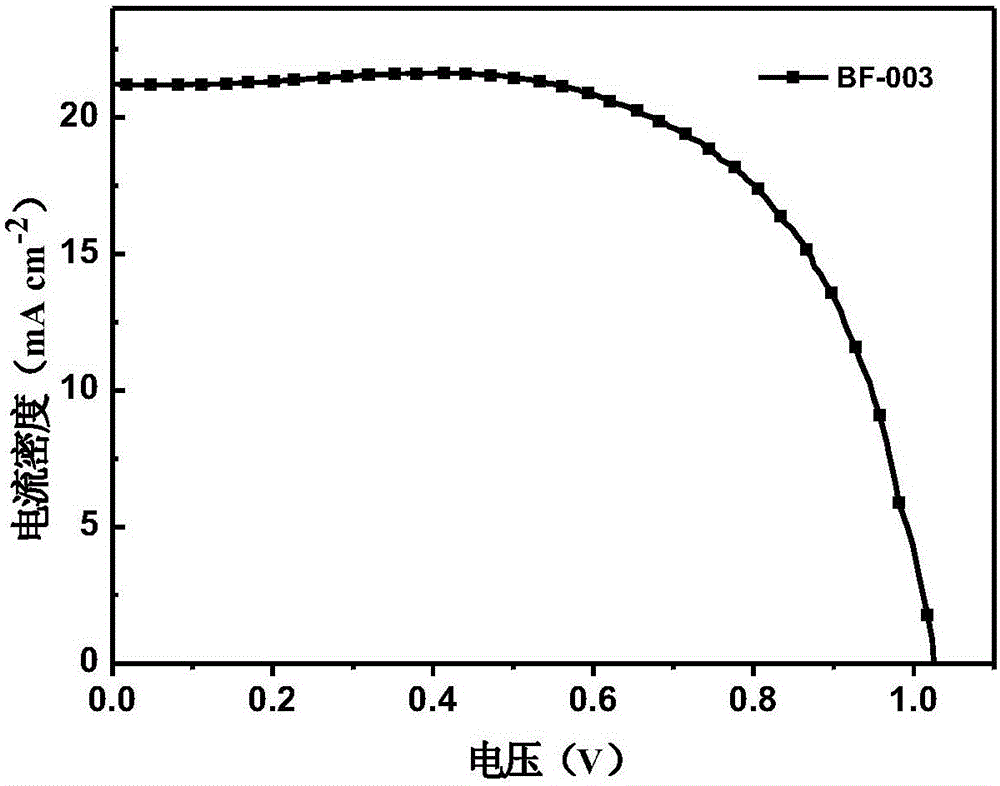
Derivative of dibenzofuran and preparation method and application thereof
A technology of dibenzofuran and derivatives, which is applied in the field of dibenzofuran derivatives and its preparation and application, and can solve the problem of low glass transition temperature of organic small molecule compounds, uncertain molecular weight of polymer materials, and difficulty in industrialization Production and other issues, to achieve the effect of favorable battery properties, high photoelectric conversion efficiency, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment one precursor 1
[0037] The reaction scheme is as follows:
[0038]
[0039] 149.6g (0.8mol) p-bromoanisole, 108.3g (0.88mol) p-methoxyaniline and 107.5g (1.2mol) potassium tert-butoxide were successively added to a 2L three-necked flask to obtain mixture I, and to mixture I was added 800g toluene, through N 2 After replacement for 10 min, 0.9 g (0.004 mol) of palladium acetate and 1.62 g (0.008 mol) of tri-tert-butylphosphine were added, and the mixture was refluxed at 110° C. for 5 hours. The reaction was quenched by adding water, separated, extracted, and the solvent was removed under reduced pressure to obtain a crude product. The crude product was recrystallized from a mixed solvent of toluene and ethyl acetate (mass ratio 3:1) to obtain 119 g of light yellow solid with a yield of 64.9%. GC-MS: C 14 h 15 NO 2 : Calculated value: 229.11, Measured value [M] + = 229.27.
Embodiment 2
[0040] The preparation of embodiment two precursor 2
[0041] The reaction scheme is as follows:
[0042]
[0043] Synthesis of Intermediate 1:
[0044] Add 20g (0.06mol) 4,4'-dibromodiphenylamine and 13.1g (0.06mol) (BOC) to a 500mL three-necked flask 2 O, mix well to obtain mixture II, add 100g THF (tetrahydrofuran) to the above mixture II, and protect with nitrogen. Add 0.75g (0.006mol) DMAP (4-dimethylaminopyridine) to the system, and react at 65°C for 2h. After the reaction was completed, the temperature was lowered to 20-25° C., and the solvent was removed under reduced pressure to obtain a brown oil, which was filtered through a silica gel column to obtain 51.6 g of a white solid with a yield of 98%. LC-MS: C 17 h 17 Br 2 NO 2 , calculated value: 424.96, measured value: [M+2] + = 427.13.
[0045] Synthesis of Intermediate 2:
[0046] Add 42.7 g (0.1 mol) of intermediate 1 and 50.4 g (0.22 mol) of precursor 1 (prepared by Example 1) into a 2 L three-necked f...
Embodiment 3
[0049] The preparation of embodiment three precursor 3
[0050] The reaction scheme is as follows:
[0051]
[0052] Synthesis of intermediate 3:
[0053] The feed ratio and preparation process are the same as the synthesis of intermediate 1. 15.1 g of white solid was obtained with a yield of 95%. LC-MS: C 17 h 12 BrNO 2 , calculated value: 422.95, measured value: [M+2] + = 425.11.
[0054] Synthesis of Intermediate 4:
[0055] Add 2.4 g (0.01 mol) of intermediate 3 and 5.0 g (0.022 mol) of precursor 1 (prepared by Example 1) into a 100 mL three-necked flask to obtain mixture V. Add 50 g of toluene to the mixture V, and protect it with nitrogen. Then add 2.4g (0.025mol) sodium tert-butoxide, 0.29g (4×10 -4 mol)Pd(dppf)Cl 2 , 0.24g (8×10 -4 mol) tri-tert-butylphosphine tetrafluoroborate, mix well, turn on the mechanical stirring, and react at 110°C for 3h. After the reaction was completed, the temperature of the system was lowered to 20-25° C., suction filtered,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com