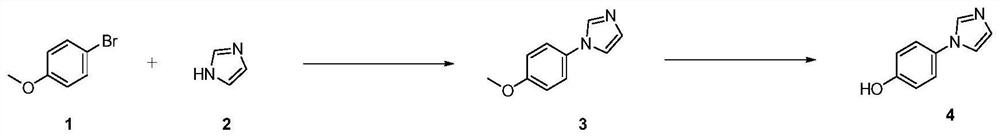
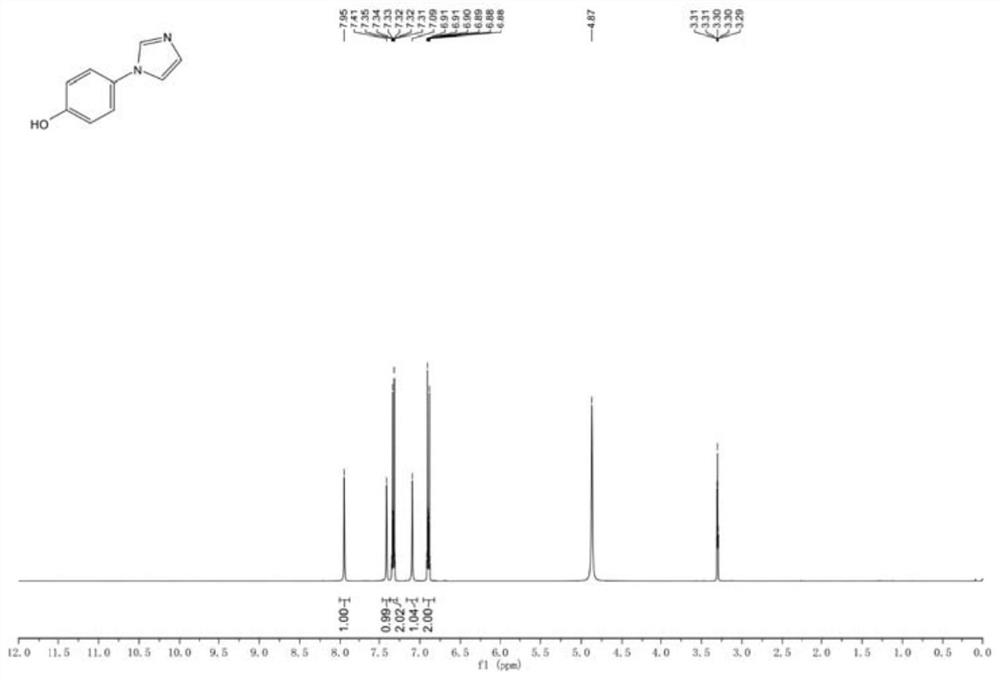
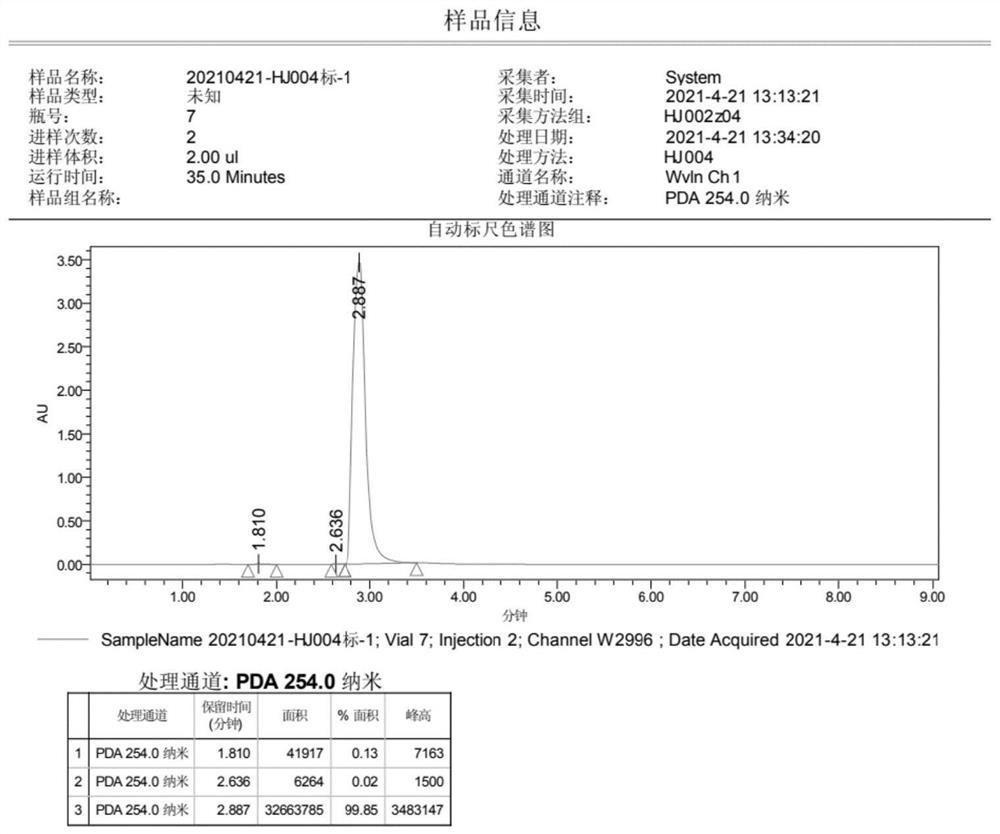
Preparation method of 4-(imidazole-1-yl) phenol
A technology of phenol and imidazole, applied in the field of preparation of 4-phenol, can solve the problems of difficult extraction, high difficulty, poor product color and the like, and achieves the effects of low price, low cost and satisfying market demand
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Step (1): In the reactor, add 1kg p-bromoanisole, 0.5kg imidazole, 70g cuprous oxide, 1kg potassium carbonate and 2LNMP, and stir. The reaction temperature was raised to 120°C and reacted for 12 hours. After the reaction is completed, filter and rinse with 500 mL of ethyl acetate to remove solids; add 2 L of 8% NaOH aqueous solution and stir, and solids are generated, and then filter to remove solids. To the liquid, ethyl acetate (3x1 L) was added for extraction, and the organic phases were combined. Add saturated brine to the organic phase, stir, separate, dry, spin off the solvent, and recrystallize using methyl tert-butyl ether to obtain 500 g of 1-(4-methoxyphenyl)-1H-imidazole; rate 53%;
[0037] Step (2): In the reactor, add 0.5kg 1-(4-methoxyphenyl)-1H-imidazole, dissolve in dichloromethane, stir, cool the system to 0°C in an ice-salt bath, and under nitrogen protection, Add 4.3L of 1.0M BBr dropwise 3 dichloromethane solution. After dropping, the temperatur...
Embodiment 2
[0041] Step (1): In the reactor, add 1kg p-bromoanisole, 0.5kg imidazole, 70g cuprous oxide, 1kg cesium carbonate and 2LNMP, and stir. The reaction temperature was raised to 120°C and reacted for 12 hours. After the reaction is completed, filter, add NaOH aqueous solution, stir, and filter. Extract with ethyl acetate, and combine the organic phases. Add saturated brine to the organic phase, stir, separate liquids, dry, spin off the solvent, and crystallize methyl tert-butyl ether to obtain 700 g of 1-(4-methoxyphenyl)-1H-imidazole; yield 74 %.
[0042] Step (2) is the same as in Example 1.
Embodiment 3
[0044] Step (1): In the reactor, add 1kg p-bromoanisole, 0.5kg imidazole, 70g cuprous iodide, 1kg cesium carbonate and 2LDMF, and stir. The reaction temperature was raised to 120°C and reacted for 12 hours. After the reaction is completed, filter, add NaOH aqueous solution, stir, and filter. Extract with ethyl acetate, and combine the organic phases. Add saturated brine to the organic phase, stir, separate liquids, dry, spin off the solvent, crystallize methyl tert-butyl ether to obtain 450 g of 1-(4-methoxyphenyl)-1H-imidazole; yield 48 %.
[0045] Step (2) is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com