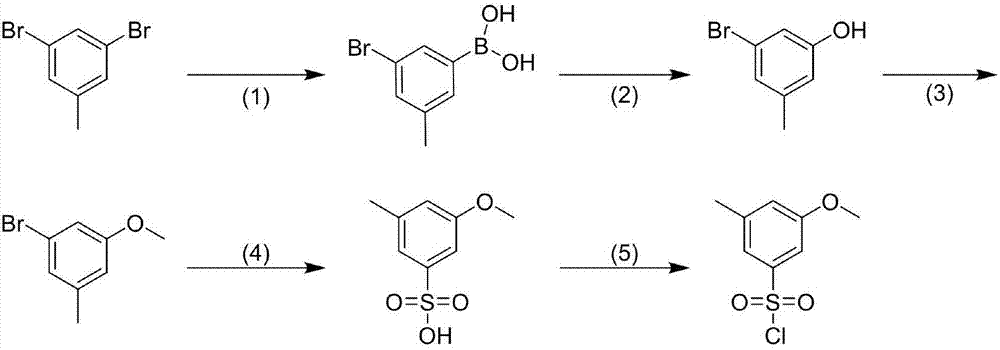
Preparation process for 3-methyl-5-methoxybenzenesulfonyl chloride
A kind of technology of methoxybenzenesulfonyl chloride and methoxybenzenesulfonic acid, which is applied in the field of preparation of 3-methyl-5-methoxybenzenesulfonyl chloride, and can solve the problem that no literature reports 3-methyl-5-methylbenzene Oxybenzenesulfonyl chloride preparation technology and other problems, to achieve the effects of good application value, high yield, and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] A. Preparation of 3-methyl-5-bromophenylboronic acid
[0014] In a three-necked flask, 3,5-dibromotoluene (20.0g, 80.0mmol) was added to dry tetrahydrofuran (100mL), under N 2 Under protection, lower the temperature to -78°C, slowly add n-butyllithium (5.1g, 80.0mmol) dropwise, keep the temperature at -78°C and stir for 30min, add triisopropyl borate (22.6g, 120.0mmol), keep the reaction for 2h . After the reaction is complete, add water to quench, adjust the pH of the solution to 6 with 2mol / L dilute hydrochloric acid, extract with ethyl acetate (50mL×3), dry over anhydrous sodium sulfate, filter, distill the filtrate to remove the solvent under reduced pressure, and beat with n-hexane (30mL) , and dried to obtain 15.4 g of 3-methyl-5-bromophenylboronic acid as a white solid. The yield was 89.5%.
[0015] 1 H NMR (400MHz, CD 3 OD) δ7.65 (s, 1H), 7.48 (s, 1H), 7.37 (s, 1H), 2.32 (s, 3H).
[0016] B. Preparation of 3-methyl-5-bromophenol
[0017] In a three-necked...
Embodiment 2
[0028] A. Preparation of 3-methyl-5-bromophenylboronic acid
[0029] In a three-necked flask, add 3,5-dibromotoluene (20.0g, 80.0mmol) into dry dimethyltetrahydrofuran (100mL), and 2 Under protection, lower the temperature to -78°C, slowly add n-butyllithium (5.1g, 80.0mmol) dropwise, keep the temperature at -78°C and stir for 30min, add triisopropyl borate (22.6g, 120.0mmol), keep the reaction for 2h . After the reaction is complete, add water to quench, adjust the pH of the solution to 6 with 2mol / L dilute hydrochloric acid, extract with ethyl acetate (50mL×3), dry over anhydrous sodium sulfate, filter, distill the filtrate to remove the solvent under reduced pressure, and beat with n-hexane (30mL) , and dried to obtain 15.2 g of 3-methyl-5-bromophenylboronic acid as a white solid. The yield was 88.6%.
[0030] 1 H NMR (400MHz, CD 3 OD) δ7.65 (s, 1H), 7.48 (s, 1H), 7.37 (s, 1H), 2.32 (s, 3H).
[0031] B. Preparation of 3-methyl-5-bromophenol
[0032] In a three-necked...
Embodiment 3
[0043] A. Preparation of 3-methyl-5-bromophenylboronic acid
[0044] In a three-necked flask, 3,5-dibromotoluene (20.0g, 80.0mmol) was added to dry tetrahydrofuran (100mL), under N 2 Under protection, lower the temperature to -78°C, slowly add tert-butyllithium (5.1g, 80.0mmol) dropwise, keep the temperature at -78°C and stir for 30min, add triisopropyl borate (22.6g, 120.0mmol), keep the reaction for 2h . After the reaction is complete, add water to quench, adjust the pH of the solution to 6 with 2mol / L dilute hydrochloric acid, extract with ethyl acetate (50mL×3), dry over anhydrous sodium sulfate, filter, distill the filtrate to remove the solvent under reduced pressure, and beat with n-hexane (30mL) , and dried to obtain 15.5 g of 3-methyl-5-bromophenylboronic acid as a white solid. The yield was 90.3%.
[0045] 1 H NMR (400MHz, CD 3 OD) δ7.65 (s, 1H), 7.48 (s, 1H), 7.37 (s, 1H), 2.32 (s, 3H).
[0046] B. Preparation of 3-methyl-5-bromophenol
[0047] In a three-nec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com