Preparation method of bisphenol monomer containing phthalazinone structure
A technology of phthalazinone and monomers, which is applied in the field of preparation of bisphenol monomers containing phthalazinone structures, can solve problems such as poor solubility, and achieve the effects of mild reaction, reduced synthesis cost, and low raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
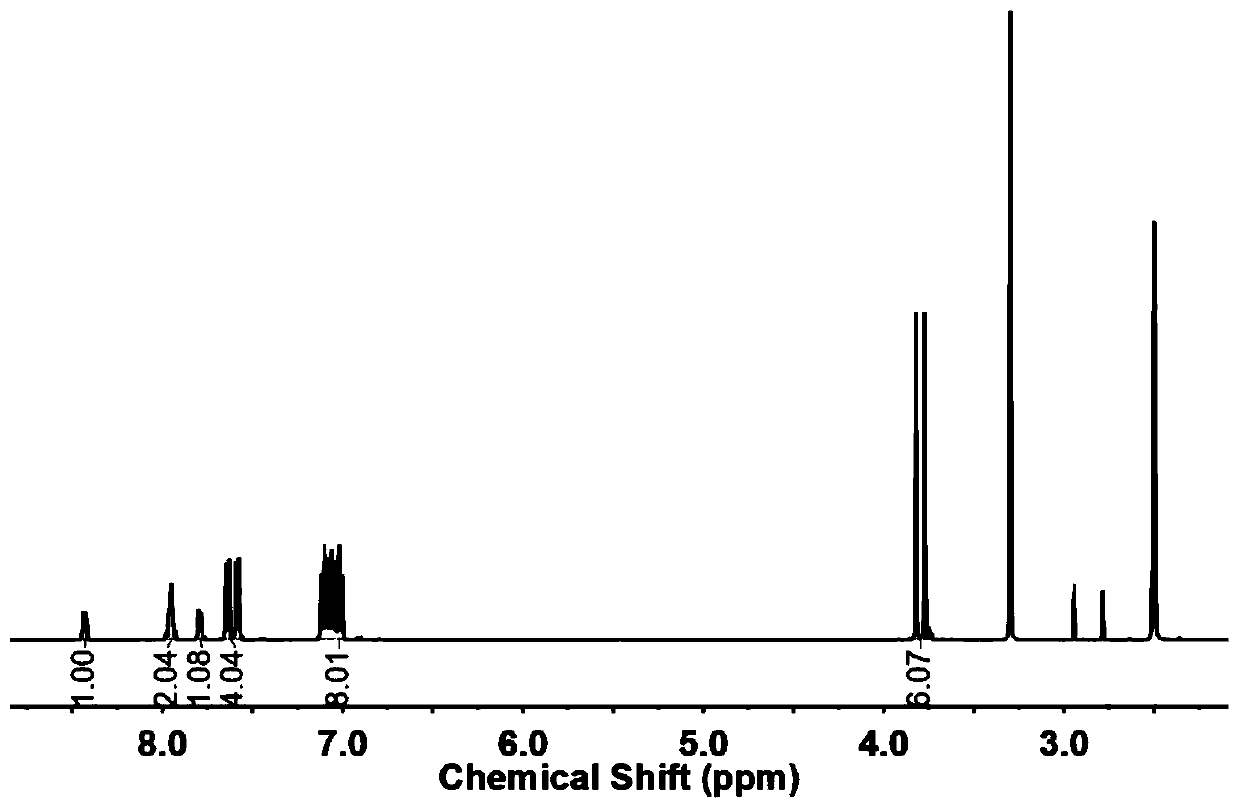
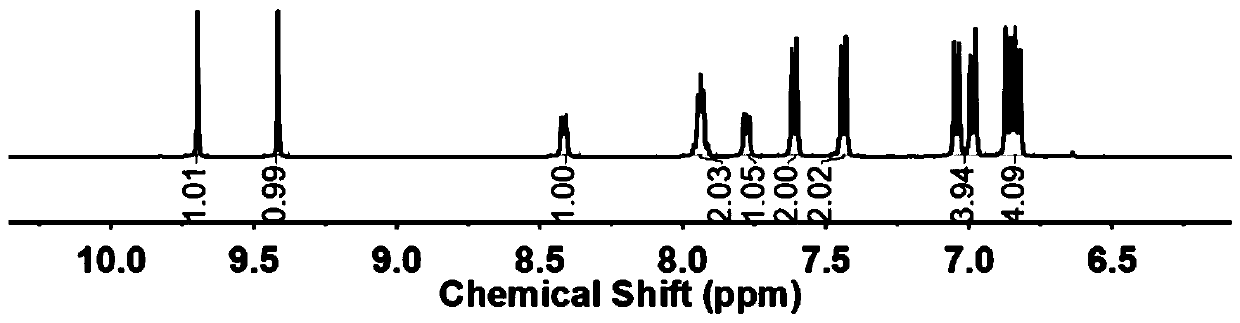
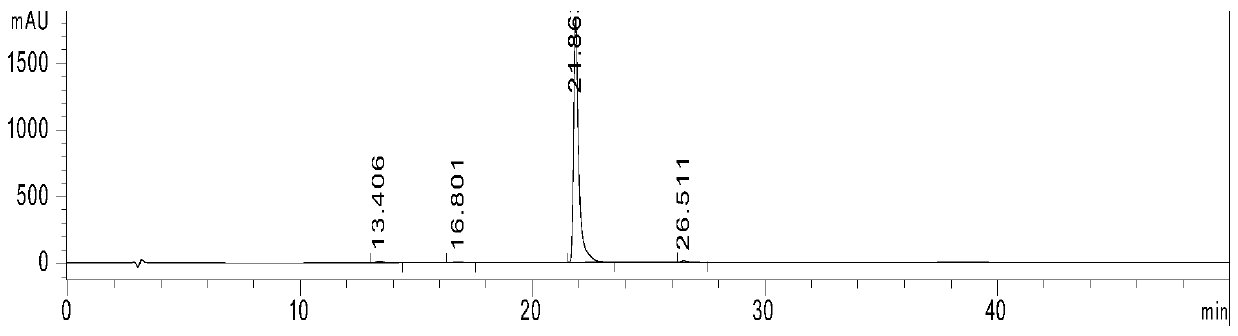
[0018] Embodiment 1: Add DHPZ and K in the there-necked flask that magnetic stirring, reflux condensation device are housed 2 CO 3 , Salt formation reaction at 135°C for 5-6 hours, after the completion of the salt formation reaction, the reaction solution was lowered to room temperature, then added Ullmann coupling ligand PNTM, catalyst CuI and reactant BPM, and reacted in the dark for 12 hours, the reaction The temperature was gradually raised from room temperature to 130°C. After the reaction, sink the reaction solution into hot water and drain it overnight, dissolve the brown solid in chloroform, add excess anhydrous sodium sulfate to the filtrate obtained by suction filtration, and obtain a brown powdery solid after rotary evaporation, and use acetone or ethanol Rinse 1-2 times to obtain a light yellow solid, which is placed in a vacuum drying oven at 100°C for 10-12 hours in vacuum. Refined by DMAc at a mass volume ratio of 1:2.5 to obtain a white solid powder.
Embodiment 2
[0019] Embodiment 2: Add DHPZ and K in the there-necked flask that magnetic stirring, reflux condensing device are housed 2 CO 3 , salt-forming reaction at 135°C for 5-6 hours. After the salt-forming reaction, the reaction liquid was lowered to room temperature, and then Ullmann coupling ligand PNTM, catalyst CuI and reactant BPM were added, and the reaction was protected from light for 18 hours. The temperature was gradually raised from room temperature to 130°C. After the reaction, sink the reaction solution into hot water and drain it overnight, dissolve the brown solid in chloroform, add excess anhydrous sodium sulfate to the filtrate obtained by suction filtration, and obtain a brown powdery solid after rotary evaporation, and use acetone or ethanol Rinse 1-2 times to obtain a light yellow solid, which is placed in a vacuum drying oven at 100°C for 10-12 hours in vacuum. Refined by DMAc at a mass volume ratio of 1:2.5 to obtain a white solid powder.
Embodiment 3
[0020] Embodiment 3: Add DHPZ and K in the there-necked flask that magnetic stirring, reflux condensing device are housed 2 CO 3 , salt-forming reaction at 135°C for 5-6 hours. After the salt-forming reaction, the reaction liquid was lowered to room temperature, and then Ullmann coupling ligand PNTM, catalyst CuI and reactant BPM were added, and the reaction was protected from light for 24 hours. The temperature was gradually raised from room temperature to 130°C. After the reaction, sink the reaction solution into hot water and drain it overnight, dissolve the brown solid in chloroform, add excess anhydrous sodium sulfate to the filtrate obtained by suction filtration, and obtain a brown powdery solid after rotary evaporation, and use acetone or ethanol Rinse 1-2 times to obtain a light yellow solid, which is placed in a vacuum drying oven at 100°C for 10-12 hours in vacuum. Refined by DMAc at a mass volume ratio of 1:2.5 to obtain a white solid powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com