Method of preparing adapalene
A compound and alkyl technology, applied in the field of pharmaceutical synthesis, can solve the problems of complicated operation, increased complexity and high cost, and achieve the effects of simplifying operation steps, increasing raw material consumption and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Synthesis of ethyl 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoate
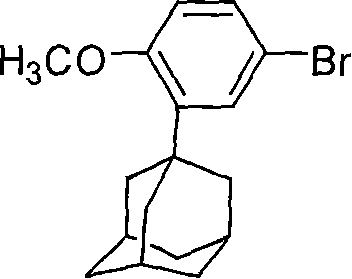
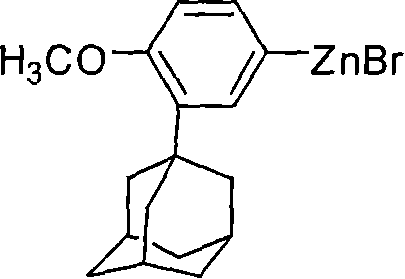
[0028] Add 6.6 grams of zinc powder, 200 milliliters of tetrahydrofuran and 2 grams of iodine in a 1000 milliliter round bottom flask, after initiating the reaction, slowly add 32 grams (0.1 moles) of 2-(1-adamantyl)-4-bromobenzyl ether, stirring to keep the reaction slightly boiling. Reflux for 3 hours, cool to room temperature, add 28 grams of ethyl 6-bromo-2-naphthoate, and 1.2gNiCl to the reaction mixture 2 / DPPE catalyst, stirred and reacted at room temperature for 2 hours. After the reaction was completed, the reaction solution was poured into 250 ml of water, extracted with dichloromethane, and the organic phase was separated. The organic phase was washed with water until neutral, dried, and recrystallized with ethyl acetate. 41.60 g of white solid was obtained with a yield of 94.5%.
Embodiment 2
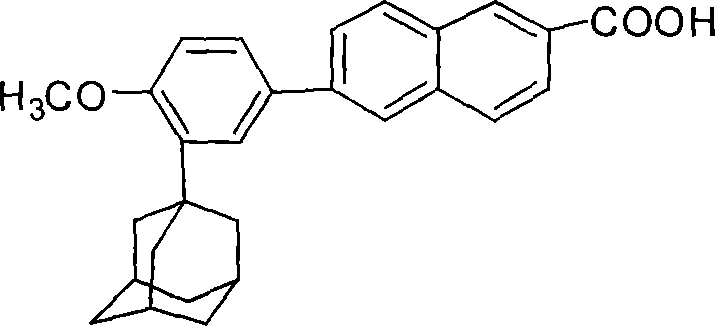
[0029] Example 2 Synthesis of 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid (adapalene)
[0030] In a 1000 ml four-necked flask, 200 ml of tetrahydrofuran solution containing 11 grams of 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid ethyl ester was added, and then mass 200 ml of 4% potassium hydroxide solution was refluxed for 3 hours. After the reaction was complete, tetrahydrofuran was distilled off. Add 250 ml of water to the residue, filter to obtain a solid, wash with water until neutral, add the solid to 300 ml of water, acidify to pH 1 with concentrated hydrochloric acid, stir for 2 hours, filter to obtain a crude product, and recrystallize the crude product from tetrahydrofuran to obtain the product 9.8 g, the yield is 95.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com