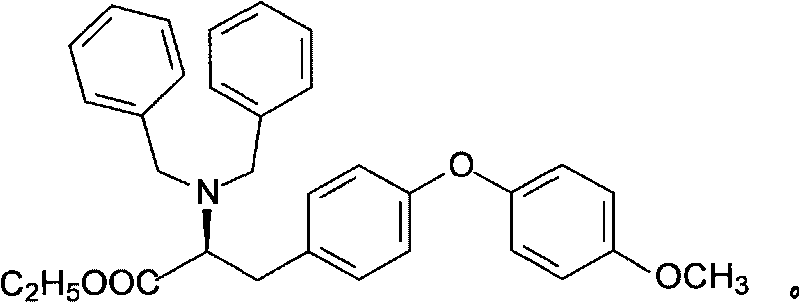
O-p-methoxyphenyl-N,N-ethyl dibenzyl-tyrosine and synthesizing method
A dibenzyl tyrosine ethyl ester and methoxyphenyl technology, which is applied in the field of synthesis of L-levothyroxine sodium, can solve the problems of many reaction by-products, harsh conditions, and low yield of the main product, etc. Effects of short time, short synthesis cycle, and reduced reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] At room temperature, p-bromoanisole (13mmol, 2.43g), N,N-dibenzyltyrosine ethyl ester (10mmol, 4.21g), potassium phosphate (13mmol, 2.75g), N,N-dimethyl Glycine hydrochloride (3.5mmol, 0.49g) and copper sulfate pentahydrate (2mmol, 0.49g) were placed in a 100mL three-necked flask filled with N,N-dimethylformamide (50mL). The suspension of the above mixture was heated and stirred at 100°C for 8 hours. After the reaction was completed and the reaction liquid was cooled to room temperature, it was filtered, an appropriate amount of water was added to the filtrate and extracted with ether, the filter cake was washed with an appropriate amount of ether, and the combined organic liquid was washed with water for 2 to 3 times and then dried overnight with anhydrous sodium sulfate. The desiccant was filtered, and the filtrate was concentrated under reduced pressure on a rotary evaporator to obtain a crude product. Chromatographic separation was carried out on silica gel column ...
Embodiment 2
[0053] At room temperature, p-bromoanisole (13mmol, 2.43g), N,N-dibenzyltyrosine ethyl ester (10mmol, 4.21g), potassium phosphate (13mmol, 2.75g), N,N-dimethyl Glycine hydrochloride (3.5mmol, 0.49g) and copper acetate monohydrate (2mmol, 0.40g) were placed in a 100mL three-necked flask filled with N,N-dimethylformamide (50mL). The suspension of the above mixture was heated and stirred at 100°C for 8 hours. The post-processing operation was the same as in Example 1 to obtain a light yellow transparent viscous liquid product. The yield was 48.5%, e.e% was 92.7%.
Embodiment 3
[0055] At room temperature, p-bromoanisole (13mmol, 2.43g), N,N-dibenzyltyrosine ethyl ester (10mmol, 4.21g), potassium phosphate (13mmol, 2.75g), N,N-dimethyl Glycine hydrochloride (3.5mmol, 0.49g) and copper sulfate pentahydrate (2mmol, 0.49g) were placed in a 100mL three-necked flask filled with N-methylpyrrolidone (50mL). The suspension of the above mixture was heated and stirred at 100°C for 8 hours. The post-processing operation was the same as in Example 1 to obtain a light yellow transparent viscous liquid product. The yield was 56.1%, e.e% was 90.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com