Synthesis method of carbonate modified fluoride-free organic phosphine ligand
A synthesis method and organic phosphine technology are applied in the synthesis field of carbonate-modified fluorine-free organic phosphine ligands, which can solve environmental problems, high price of fluorocarbon fluorine-containing ligands, etc. The effect of using
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
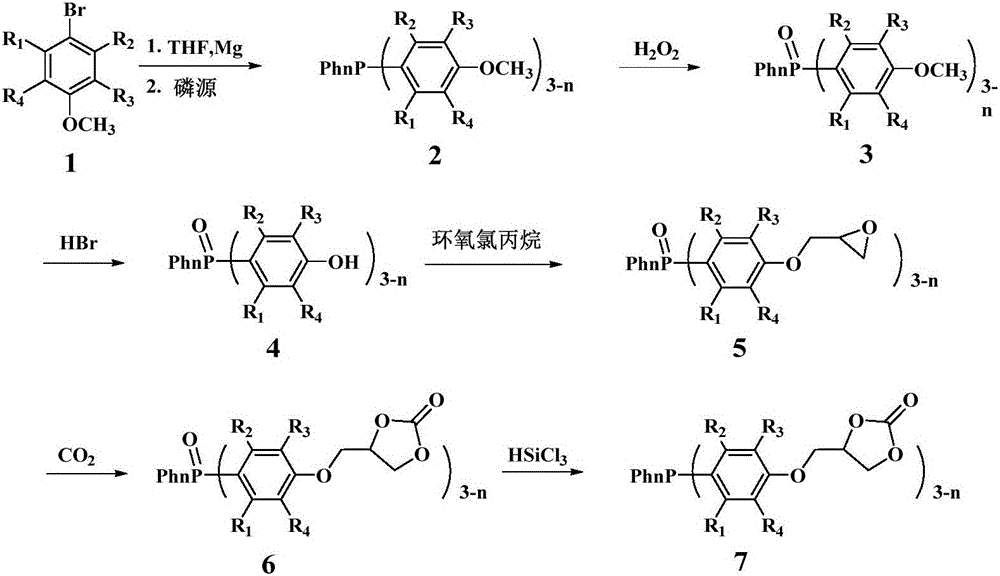
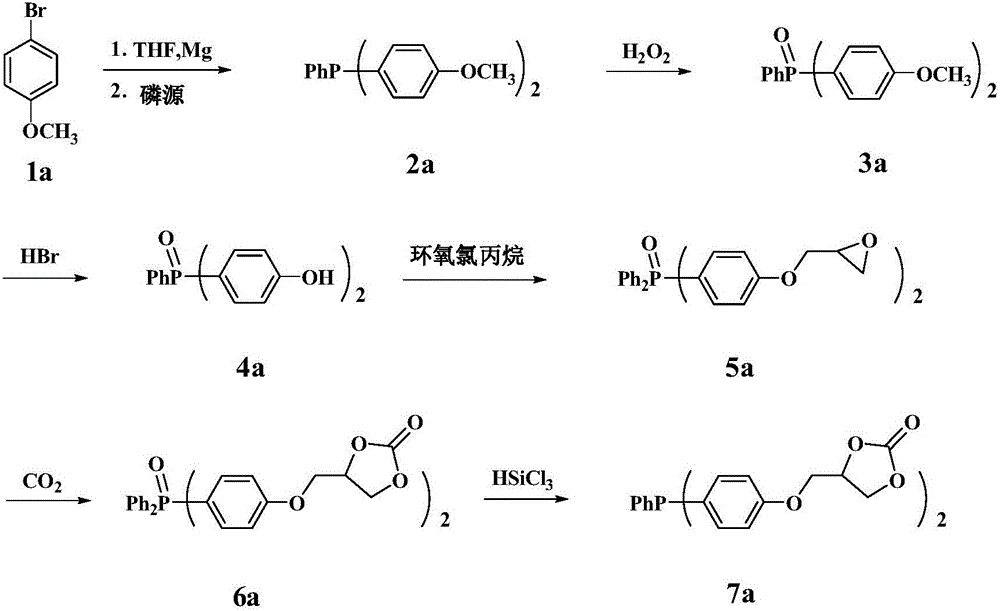
Embodiment 1
[0026] The first step, the synthesis of compound 2a (two (4-methoxyphenyl) phenylphosphine)
[0027] The magnesium powder was washed with hydrochloric acid and acetone respectively, and sucked dry. THF was refluxed with metal Na until the benzophenone turned dark purple. Add fresh Mg powder into the reaction device, N 2 Replaced 3 times, followed by adding I 2 pellets and refined THF. N 2 Under protection, the dried compound 1a (4-bromoanisole) was transferred to the dropping funnel. The reaction was initiated by heating, and a THF solution of 4-bromoanisole was slowly added dropwise. After the dropwise addition was completed, the mixture was stirred at room temperature for 1 h to obtain a dark black mixture. Phenyl phosphorus dichloride was slowly added dropwise to the Grignard reagent, and after the addition was complete, stirred for 2h. The reaction mixture was slowly added to 10% glacial hydrochloric acid aqueous solution, extracted with ether, and the solvent was d...
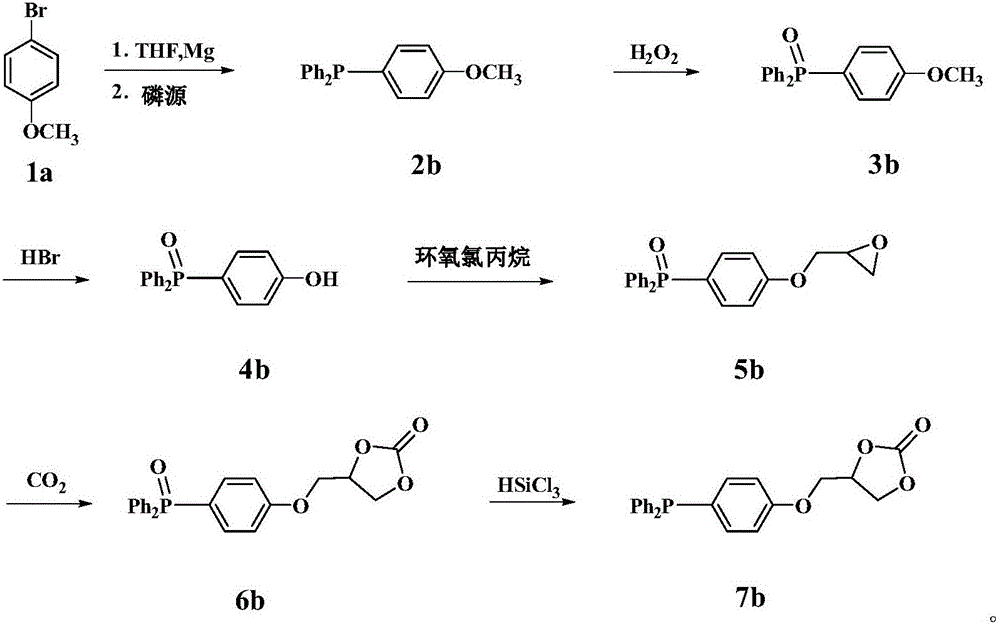
Embodiment 2
[0041] The first step, the synthesis of compound 2b ((4-methoxyphenyl) diphenylphosphine)
[0042] The magnesium powder was washed with hydrochloric acid and acetone respectively, and sucked dry. THF was refluxed with metal Na until the benzophenone turned dark purple. Add the newly prepared Mg powder into the dropwise reflux reaction device, and then use N 2 Replaced 3 times, followed by adding I 2 pellets and refined THF, in N 2 Under protection, the dried compound 1a (4-bromoanisole) was transferred to the dropping funnel. The reaction was initiated by heating, and a THF solution of 4-bromoanisole was slowly added dropwise. After the dropwise addition was completed, the mixture was stirred at room temperature for 1 h to obtain a dark black mixture. Slowly add diphenylphosphorus chloride dropwise to the Grignard reagent, after the addition is complete, stir for 2h. The reaction mixture was slowly added to 10% aqueous glacial hydrochloric acid, extracted with dichlorome...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com